Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia
- PMID: 31974170
- PMCID: PMC7124979
- DOI: 10.1158/2159-8290.CD-19-0710
Monocytic Subclones Confer Resistance to Venetoclax-Based Therapy in Patients with Acute Myeloid Leukemia
Abstract
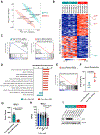
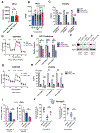
Venetoclax-based therapy can induce responses in approximately 70% of older previously untreated patients with acute myeloid leukemia (AML). However, up-front resistance as well as relapse following initial response demonstrates the need for a deeper understanding of resistance mechanisms. In the present study, we report that responses to venetoclax +azacitidine in patients with AML correlate closely with developmental stage, where phenotypically primitive AML is sensitive, but monocytic AML is more resistant. Mechanistically, resistant monocytic AML has a distinct transcriptomic profile, loses expression of venetoclax target BCL2, and relies on MCL1 to mediate oxidative phosphorylation and survival. This differential sensitivity drives a selective process in patients which favors the outgrowth of monocytic subpopulations at relapse. Based on these findings, we conclude that resistance to venetoclax + azacitidine can arise due to biological properties intrinsic to monocytic differentiation. We propose that optimal AML therapies should be designed so as to independently target AML subclones that may arise at differing stages of pathogenesis. SIGNIFICANCE: Identifying characteristics of patients who respond poorly to venetoclax-based therapy and devising alternative therapeutic strategies for such patients are important topics in AML. We show that venetoclax resistance can arise due to intrinsic molecular/metabolic properties of monocytic AML cells and that such properties can potentially be targeted with alternative strategies.
©2020 American Association for Cancer Research.
Conflict of interest statement
Figures



References
-
- Buchner T, Berdel WE, Haferlach C, Haferlach T, Schnittger S, Muller-Tidow C, et al. Age-related risk profile and chemotherapy dose response in acute myeloid leukemia: a study by the German Acute Myeloid Leukemia Cooperative Group. J Clin Oncol 2009;27(1):61–9 doi 10.1200/JCO.2007.15.4245. - DOI - PubMed
-
- Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 2009;10(3):223–32 doi 10.1016/S1470-2045(09)70003-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

