Desialylation of O-glycans on glycoprotein Ibα drives receptor signaling and platelet clearance
- PMID: 31974202
- PMCID: PMC7776245
- DOI: 10.3324/haematol.2019.240440
Desialylation of O-glycans on glycoprotein Ibα drives receptor signaling and platelet clearance
Abstract
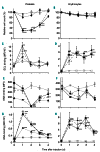
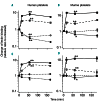
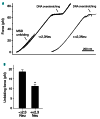
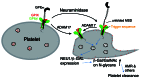
During infection neuraminidase desialylates platelets and induces their rapid clearance from circulation. The underlying molecular basis, particularly the role of platelet glycoprotein (GP)Ibα therein, is not clear. Utilizing genetically altered mice we report that the extracellular domain of GPIbα, but neither von Willebrand factor nor ADAM17 (a disintegrin and metalloprotease 17), is required for platelet clearance induced by intravenous injection of neuraminidase. Lectin binding to platelets following neuraminidase injection over time revealed that the extent of desialylation of O-glycans correlates with the decrease of platelet count in mice. Injection of α2,3-neuraminidase reduces platelet counts in wild-type but not in transgenic mice expressing only a chimeric GPIbα that misses most of its extracellular domain. Neuraminidase treatment induces unfolding of the O-glycosylated mechanosensory domain in GPIbα as monitored by single-molecule force spectroscopy, increases the exposure of the ADAM17 shedding cleavage site in the mechanosensory domain on the platelet surface, and induces ligand-independent GPIb-IX signaling in human and murine platelets. These results suggest that desialylation of O-glycans of GPIbα induces unfolding of the mechanosensory domain, subsequent GPIb-IX signaling including amplified desialylation of N-glycans, and eventually rapid platelet clearance. This new molecular mechanism of GPIbα-facilitated clearance could potentially resolve many puzzling and seemingly contradicting observations associated with clearance of desialylated or hyposialylated platelets.
Figures


Comment in
-
New insights into glycoprotein Ibα desialylation-mediated platelet clearance.Platelets. 2020 Jul 3;31(5):621-623. doi: 10.1080/09537104.2020.1764922. Epub 2020 Jun 4. Platelets. 2020. PMID: 32498669 Free PMC article. No abstract available.
References
-
- Choi SI, Simone JV, Jorney LJ. Neuraminidase-induced thrombocytopenia in rats. Br J Haematol. 1972;22(1):93-101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

