Alternative activation of human macrophages enhances tissue factor expression and production of extracellular vesicles
- PMID: 31974204
- PMCID: PMC7849567
- DOI: 10.3324/haematol.2019.220210
Alternative activation of human macrophages enhances tissue factor expression and production of extracellular vesicles
Abstract
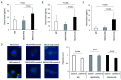
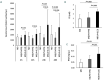
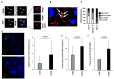
Macrophages are versatile cells that can be polarized by the tissue environment to fulfill required needs. Proinflammatory polarization is associated with increased tissue degradation and propagation of inflammation whereas alternative polarization within a Th2 cytokine environment is associated with wound healing and angiogenesis. To understand if polarization of macrophages can lead to a procoagulant macrophage subset we polarized human monocyte derived macrophages to a proinflammatory and an alternative activation state. Alternative polarization with interleukin-4 and IL-13 led to a macrophage phenotype characterized by increased tissue factor (TF) production and release and by an increase in extracellular vesicle production. In addition, also TF activity was enhanced in extracellular vesicles of alternatively polarized macrophages. This TF induction was dependent on signal transducer and activator of transcription-6 signaling and poly ADP ribose polymerase activity. In contrast to monocytes, human macrophages did not show increased tissue factor expression upon stimulation with lipopolysaccharide and interferon-γ. Previous polarization to either a proinflammatory or an alternative activation subset does not change the subsequent stimulation of TF. The inability of proinflammatory activated macrophages to respond to lipopolysaccharide and interferon-γ with an increase in TF production seems to be due to an increase in TF promoter methylation and was reversible when treating these macrophages with a demethylation agent. In conclusion, we provide evidence that proinflammatory polarization of macrophages does not lead to enhanced procoagulatory function, whereas alternative polarization of macrophages leads to an increased expression of TF and increased production of TF bearing extracellular vesicles by these cells suggesting a procoagulatory phenotype of alternatively polarized macrophages.
Figures



References
-
- Cybulsky MI, Cheong C, Robbins CS. Macrophages and dendritic cells: partners in atherogenesis. Circ Res. 2016;118(4):637-652. - PubMed
-
- Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541-66. - PubMed
-
- Lech M, Anders HJ. Macrophages and fibrosis: how resident and infiltrating mononuclear phagocytes orchestrate all phases of tissue injury and repair. Biochim Biophys Acta. 2013;1832(7):989-997. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

