Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression
- PMID: 31974313
- PMCID: PMC7022213
- DOI: 10.1073/pnas.1914655117
Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression
Abstract
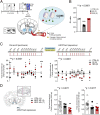
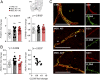
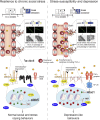
Preclinical and clinical studies suggest that inflammation and vascular dysfunction contribute to the pathogenesis of major depressive disorder (MDD). Chronic social stress alters blood-brain barrier (BBB) integrity through loss of tight junction protein claudin-5 (cldn5) in male mice, promoting passage of circulating proinflammatory cytokines and depression-like behaviors. This effect is prominent within the nucleus accumbens, a brain region associated with mood regulation; however, the mechanisms involved are unclear. Moreover, compensatory responses leading to proper behavioral strategies and active resilience are unknown. Here we identify active molecular changes within the BBB associated with stress resilience that might serve a protective role for the neurovasculature. We also confirm the relevance of such changes to human depression and antidepressant treatment. We show that permissive epigenetic regulation of cldn5 expression and low endothelium expression of repressive cldn5-related transcription factor foxo1 are associated with stress resilience. Region- and endothelial cell-specific whole transcriptomic analyses revealed molecular signatures associated with stress vulnerability vs. resilience. We identified proinflammatory TNFα/NFκB signaling and hdac1 as mediators of stress susceptibility. Pharmacological inhibition of stress-induced increase in hdac1 activity rescued cldn5 expression in the NAc and promoted resilience. Importantly, we confirmed changes in HDAC1 expression in the NAc of depressed patients without antidepressant treatment in line with CLDN5 loss. Conversely, many of these deleterious CLDN5-related molecular changes were reduced in postmortem NAc from antidepressant-treated subjects. These findings reinforce the importance of considering stress-induced neurovascular pathology in depression and provide therapeutic targets to treat this mood disorder and promote resilience.
Keywords: antidepressant; epigenetic; inflammation; mood disorders; vascular.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Wohleb E. S., Franklin T., Iwata M., Duman R. S., Integrating neuroimmune systems in the neurobiology of depression. Nat. Rev. Neurosci. 17, 497–511 (2016). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

