Neuropeptide receptor genes GHSR and NMUR1 are candidate epigenetic biomarkers and predictors for surgically treated patients with oropharyngeal cancer
- PMID: 31974445
- PMCID: PMC6978330
- DOI: 10.1038/s41598-020-57920-z
Neuropeptide receptor genes GHSR and NMUR1 are candidate epigenetic biomarkers and predictors for surgically treated patients with oropharyngeal cancer
Abstract
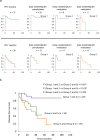
Pathological staging and histological grading systems are useful, but imperfect, predictors of recurrence in head and neck squamous cell carcinoma (HNSCC). Aberrant promoter methylation is the main type of epigenetic modification that plays a role in the inactivation of tumor suppressor genes. To identify new potential prognostic markers, we investigated the promoter methylation status of five neuropeptide receptor genes. The methylation status of the target genes was compared with clinical characteristics in 278 cases; 72 hypopharyngeal cancers, 54 laryngeal cancers, 75 oropharyngeal cancers, and 77 oral cavity cancers were studied. We found that the NTSR1, NTSR2, GHSR, MLNR, and NMUR1 promoters were methylated in 47.8%, 46.8%, 54.3%, 39.2%, and 43.5% of the samples, respectively. GHSR and NMUR1 promoter methylation independently predicted recurrence in HNSCC. In patients with oropharyngeal cancer (n = 75), GHSR and NMUR1 promoter methylation significantly correlates with survival in surgically treated patients. We classified our patients as having a low, intermediate, or high-risk of death based on three factors: HPV status, and GHSR and NMUR1 promoter methylation. The disease-free survival (DFS) rates were 87.1%, 42.7%, and 17.0%, respectively. Combined data analysis of the methylation status of ten-eleven translocation (TET) family genes indicated a trend toward greater methylation indices as the number of TET methylation events increased. In the current study, we presented the relationship between the methylation status of the GHSR and NMUR1 genes and recurrence in HNSCC, specifically in risk classification of oropharyngeal carcinomas cases with HPV status.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Hashibe M, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive. Oncology. 2009;18:541–550. doi: 10.1158/1055-9965.EPI-08-0347. - DOI - PMC - PubMed
-
- Kimple RJ, et al. Development and characterization of HPV-positive and HPV-negative head and neck squamous cell carcinoma tumorgrafts. Clinical cancer research: an official journal of the American Association for. Cancer Research. 2013;19:855–864. doi: 10.1158/1078-0432.CCR-12-2746. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

