Pifithrin-α alters p53 post-translational modifications pattern and differentially inhibits p53 target genes
- PMID: 31974452
- PMCID: PMC6978515
- DOI: 10.1038/s41598-020-58051-1
Pifithrin-α alters p53 post-translational modifications pattern and differentially inhibits p53 target genes
Abstract
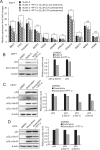
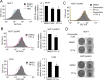
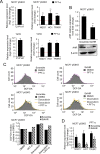
Pifithrin-α (PFT-α) is a small molecule which has been widely used as a specific inhibitor of p53 transcription activity. However, its molecular mechanism of action remains unclear. PFT-α has also been described to display potent p53-independent activity in cells. In this study, we addressed the mechanism of action of PFT-α. We found that PFT-α failed to prevent the effects of Mdm2 inhibitor Nutlin-3 on cell cycle and apoptosis in several cancer cell lines. However, PFT-α rescued normal primary fibroblasts from growth inhibition by Nutlin-3. PFT-α displayed a very limited effect on p53-dependent transcription upon its activation by Nutlin-3. Moreover, PFT-α inhibitory effect on transcription was highly dependent on the nature of the p53 target gene. PFT-α attenuated post-translational modifications of p53 without affecting total p53 protein level. Finally, we found that PFT-α can decrease the level of intracellular reactive oxygen species through activation of an aryl hydrocarbon receptor (AHR)-Nrf2 axis in a p53-independent manner. In conclusion, PFT-α inhibits only some aspects of p53 function, therefore it should be used with extreme caution to study p53-dependent processes.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

