Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli
- PMID: 31974490
- PMCID: PMC6978316
- DOI: 10.1038/s42003-020-0761-3
Development of chimeric peptides to facilitate the neutralisation of lipopolysaccharides during bactericidal targeting of multidrug-resistant Escherichia coli
Abstract
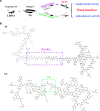
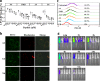
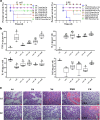
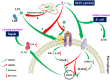
Pathogenic Escherichia coli can cause fatal diarrheal diseases in both animals and humans. However, no antibiotics or antimicrobial peptides (AMPs) can adequately kill resistant bacteria and clear bacterial endotoxin, lipopolysaccharide (LPS) which leads to inflammation and sepsis. Here, the LPS-targeted smart chimeric peptides (SCPs)-A6 and G6 are generated by connecting LPS-targeting peptide-LBP14 and killing domain-N6 via different linkers. Rigid and flexible linkers retain the independent biological activities from each component. SCPs-A6 and G6 exert low toxicity and no bacterial resistance, and they more rapidly kill multiple-drug-resistant E. coli and more effectively neutralize LPS toxicity than N6 alone. The SCPs can enhance mouse survival more effectively than N6 or polymyxin B and alleviate lung injuries by blocking mitogen-activated protein kinase and nuclear factor kappa-B p65 activation. These findings uniquely show that SCPs-A6 and G6 may be promising dual-function candidates as improved antibacterial and anti-endotoxin agents to treat bacterial infection and sepsis.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Peptides with dual mode of action: Killing bacteria and preventing endotoxin-induced sepsis.Biochim Biophys Acta. 2016 May;1858(5):971-9. doi: 10.1016/j.bbamem.2016.01.011. Epub 2016 Jan 20. Biochim Biophys Acta. 2016. PMID: 26801369 Review.
-
A cleavable chimeric peptide with targeting and killing domains enhances LPS neutralization and antibacterial properties against multi-drug resistant E. coli.Commun Biol. 2023 Nov 16;6(1):1170. doi: 10.1038/s42003-023-05528-0. Commun Biol. 2023. PMID: 37973936 Free PMC article.
-
A Novel Peptide Antibiotic, Pro10-1D, Designed from Insect Defensin Shows Antibacterial and Anti-Inflammatory Activities in Sepsis Models.Int J Mol Sci. 2020 Aug 27;21(17):6216. doi: 10.3390/ijms21176216. Int J Mol Sci. 2020. PMID: 32867384 Free PMC article.
-
Antimicrobial Peptide CMA3 Derived from the CA-MA Hybrid Peptide: Antibacterial and Anti-inflammatory Activities with Low Cytotoxicity and Mechanism of Action in Escherichia coli.Antimicrob Agents Chemother. 2015 Nov 9;60(1):495-506. doi: 10.1128/AAC.01998-15. Print 2016 Jan. Antimicrob Agents Chemother. 2015. PMID: 26552969 Free PMC article.
-
NMR Structures and Interactions of Antimicrobial Peptides with Lipopolysaccharide: Connecting Structures to Functions.Curr Top Med Chem. 2016;16(1):4-15. doi: 10.2174/1568026615666150703121943. Curr Top Med Chem. 2016. PMID: 26139110 Review.
Cited by
-
New Evidence for Artemisia absinthium as an Alternative to Classical Antibiotics: Chemical Analysis of Phenolic Compounds, Screening for Antimicrobial Activity.Int J Mol Sci. 2023 Jul 27;24(15):12044. doi: 10.3390/ijms241512044. Int J Mol Sci. 2023. PMID: 37569422 Free PMC article.
-
Thanatin: An Emerging Host Defense Antimicrobial Peptide with Multiple Modes of Action.Int J Mol Sci. 2021 Feb 3;22(4):1522. doi: 10.3390/ijms22041522. Int J Mol Sci. 2021. PMID: 33546369 Free PMC article. Review.
-
Postbiotic-Based Extracts from Native Probiotic Strains: A Promising Strategy for Food Preservation and Antimicrobial Defense.Antibiotics (Basel). 2025 Mar 18;14(3):318. doi: 10.3390/antibiotics14030318. Antibiotics (Basel). 2025. PMID: 40149128 Free PMC article.
-
Self-assembly antimicrobial peptide for treatment of multidrug-resistant bacterial infection.J Nanobiotechnology. 2024 Oct 30;22(1):668. doi: 10.1186/s12951-024-02896-5. J Nanobiotechnology. 2024. PMID: 39478570 Free PMC article.
-
BamA-targeted antimicrobial peptide design for enhanced efficacy and reduced toxicity.Amino Acids. 2023 Oct;55(10):1317-1331. doi: 10.1007/s00726-023-03307-z. Epub 2023 Sep 5. Amino Acids. 2023. PMID: 37670010
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

