Fusion of the Cas9 endonuclease and the VirD2 relaxase facilitates homology-directed repair for precise genome engineering in rice
- PMID: 31974493
- PMCID: PMC6978410
- DOI: 10.1038/s42003-020-0768-9
Fusion of the Cas9 endonuclease and the VirD2 relaxase facilitates homology-directed repair for precise genome engineering in rice
Abstract
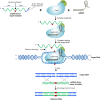
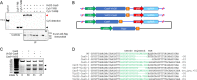
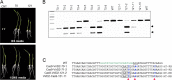
Precise genome editing by systems such as clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9 (Cas9) requires high-efficiency homology-directed repair (HDR). Different technologies have been developed to improve HDR but with limited success. Here, we generated a fusion between the Cas9 endonuclease and the Agrobacterium VirD2 relaxase (Cas9-VirD2). This chimeric protein combines the functions of Cas9, which produces targeted and specific DNA double-strand breaks (DSBs), and the VirD2 relaxase, which brings the repair template in close proximity to the DSBs, to facilitate HDR. We successfully employed our Cas9-VirD2 system for precise ACETOLACTATE SYNTHASE (OsALS) allele modification to generate herbicide-resistant rice (Oryza sativa) plants, CAROTENOID CLEAVAGE DIOXYGENASE-7 (OsCCD7) to engineer plant architecture, and generate in-frame fusions with the HA epitope at HISTONE DEACETYLASE (OsHDT) locus. The Cas9-VirD2 system expands our ability to improve agriculturally important traits in crops and opens new possibilities for precision genome engineering across diverse eukaryotic species.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

