Silencing E3 Ubiqutin ligase ITCH as a potential therapy to enhance chemotherapy efficacy in p53 mutant neuroblastoma cells
- PMID: 31974512
- PMCID: PMC6978385
- DOI: 10.1038/s41598-020-57854-6
Silencing E3 Ubiqutin ligase ITCH as a potential therapy to enhance chemotherapy efficacy in p53 mutant neuroblastoma cells
Abstract
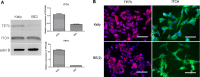
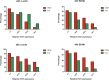
P53 mutations are responsible for drug-resistance of tumour cells which impacts on the efficacy of treatment. Alternative tumour suppressor pathways need to be explored to treat p53- deficient tumours. The E3 ubiquitin ligase, ITCH, negatively regulates the tumour suppressor protein TP73, providing a therapeutic target to enhance the sensitivity of the tumour cells to the treatment. In the present study, two p53-mutant neuroblastoma cell lines were used as in vitro models. Using immunostaining, western blot and qPCR methods, we firstly identified that ITCH was expressed on p53-mutant neuroblastoma cell lines. Transfection of these cell lines with ITCH siRNA could effectively silence the ITCH expression, and result in the stabilization of TP73 protein, which mediated the apoptosis of the neuroblastoma cells upon irradiation treatment. Finally, in vivo delivery of the ITCH siRNA using nanoparticles to the neuroblastoma xenograft mouse model showed around 15-20% ITCH silencing 48 hours after transfection. Our data suggest that ITCH could be silenced both in vitro and in vivo using nanoparticles, and silencing of ITCH sensitizes the tumour cells to irradiation treatment. This strategy could be further explored to combine the chemotherapy/radiotherapy treatment to enhance the therapeutic effects on p53-deficient neuroblastoma.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
The ubiquitin E3 ligase ITCH enhances breast tumor progression by inhibiting the Hippo tumor suppressor pathway.Oncotarget. 2014 Nov 15;5(21):10886-900. doi: 10.18632/oncotarget.2540. Oncotarget. 2014. PMID: 25350971 Free PMC article.
-
Specific activation of microRNA106b enables the p73 apoptotic response in chronic lymphocytic leukemia by targeting the ubiquitin ligase Itch for degradation.Blood. 2009 Apr 16;113(16):3744-53. doi: 10.1182/blood-2008-09-178707. Epub 2008 Dec 18. Blood. 2009. PMID: 19096009 Free PMC article.
-
Inhibitors of histone deacetylase (HDAC) restore the p53 pathway in neuroblastoma cells.Br J Pharmacol. 2008 Feb;153(4):657-68. doi: 10.1038/sj.bjp.0707608. Epub 2007 Dec 3. Br J Pharmacol. 2008. PMID: 18059320 Free PMC article.
-
p53 family: Therapeutic targets in neuroblastoma.Future Oncol. 2010 Mar;6(3):429-44. doi: 10.2217/fon.09.176. Future Oncol. 2010. PMID: 20222799 Review.
-
Neuroblastoma: oncogenic mechanisms and therapeutic exploitation of necroptosis.Cell Death Dis. 2015 Dec 3;6(12):e2010. doi: 10.1038/cddis.2015.354. Cell Death Dis. 2015. PMID: 26633716 Free PMC article. Review.
Cited by
-
Targeting Intracranial Tumours with a Combination of RNA and Chemotherapy.Pharmaceutics. 2024 Jun 18;16(6):829. doi: 10.3390/pharmaceutics16060829. Pharmaceutics. 2024. PMID: 38931949 Free PMC article.
-
Exploring the Roles of HERC2 and the NEDD4L HECT E3 Ubiquitin Ligase Subfamily in p53 Signaling and the DNA Damage Response.Front Oncol. 2021 Mar 31;11:659049. doi: 10.3389/fonc.2021.659049. eCollection 2021. Front Oncol. 2021. PMID: 33869064 Free PMC article. Review.
-
AF1q is a universal marker of neuroblastoma that sustains N-Myc expression and drives tumorigenesis.Oncogene. 2024 Apr;43(16):1203-1213. doi: 10.1038/s41388-024-02980-y. Epub 2024 Feb 27. Oncogene. 2024. PMID: 38413795 Free PMC article.
-
UBE4B interacts with the ITCH E3 ubiquitin ligase to induce Ku70 and c-FLIPL polyubiquitination and enhanced neuroblastoma apoptosis.Cell Death Dis. 2023 Nov 13;14(11):739. doi: 10.1038/s41419-023-06252-7. Cell Death Dis. 2023. PMID: 37957138 Free PMC article.
-
Non-Viral Carriers for Nucleic Acids Delivery: Fundamentals and Current Applications.Life (Basel). 2023 Mar 29;13(4):903. doi: 10.3390/life13040903. Life (Basel). 2023. PMID: 37109432 Free PMC article. Review.
References
-
- Keshelava N, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res. 2001;61:6185–6193. - PubMed
-
- Tweddle DA, Malcolm AJ, Bown N, Pearson AD, Lunec J. Evidence for the development of p53 mutations after cytotoxic therapy in a neuroblastoma cell line. Cancer Res. 2001;61:8–13. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

