MicroRNA-92b-3p is a prognostic oncomiR that targets TSC1 in clear cell renal cell carcinoma
- PMID: 31975504
- PMCID: PMC7156823
- DOI: 10.1111/cas.14325
MicroRNA-92b-3p is a prognostic oncomiR that targets TSC1 in clear cell renal cell carcinoma
Abstract
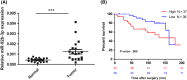
Although several studies have reported that microRNA (miR)-92b-3p is involved in various cellular processes related to carcinogenesis, its physiological role in clear cell renal cell carcinoma (ccRCC) remains unclear. To clarify the role of miR-92b-3p in ccRCC, we compared miR-92b-3p expression levels in ccRCC tissues and adjacent normal renal tissues. Significant upregulation of miR-92b-3p was observed in ccRCC tissues. Overexpression of miR-92b-3p using a miRNA mimic promoted proliferation, migration, and invasion activities of ACHN cells. Functional inhibition of miR-92b-3p by a hairpin miRNA inhibitor suppressed Caki-2 cell growth and invasion activities in vitro. Mechanistically, it was found that miR-92b-3p directly targeted the TSC1 gene, a known upstream regulator of mTOR. Overexpression of miR-92b-3p decreased the protein expression of TSC1 and enhanced the downstream phosphorylation of p70S6 kinase, suggesting that the mTOR signaling pathway was activated by miR-92b-3p in RCC cells. Importantly, a multivariate Cox proportion hazard model, based on TNM staging and high levels of miR-92b-3p, revealed that miR-92b-3p expression (high vs. low hazard ratio, 2.86; 95% confidence interval, 1.20-6.83; P = .018) was a significant prognostic factor for overall survival of ccRCC patients with surgical management. Taken together, miR-92b-3p was found to act as an oncomiR, promoting cell proliferation by downregulating TSC1 in ccRCC.
Keywords: TSC1; ccRCC; miR-92b-3p; oncomiR; proliferation.
© 2020 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
The authors have no conflicts of interest.
Figures




Similar articles
-
miR-92b-3p-TSC1 axis is critical for mTOR signaling-mediated vascular smooth muscle cell proliferation induced by hypoxia.Cell Death Differ. 2019 Sep;26(9):1782-1795. doi: 10.1038/s41418-018-0243-z. Epub 2018 Dec 5. Cell Death Differ. 2019. PMID: 30518907 Free PMC article.
-
MiR-337-3p suppresses the proliferation and metastasis of clear cell renal cell carcinoma cells via modulating Capn4.Cancer Biomark. 2018;23(4):515-525. doi: 10.3233/CBM-181645. Cancer Biomark. 2018. PMID: 30452399
-
Up-regulation of miR-181a in clear cell renal cell carcinoma is associated with lower KLF6 expression, enhanced cell proliferation, accelerated cell cycle transition, and diminished apoptosis.Urol Oncol. 2018 Mar;36(3):93.e23-93.e37. doi: 10.1016/j.urolonc.2017.09.019. Epub 2017 Oct 21. Urol Oncol. 2018. PMID: 29066014
-
miRNAs in Prediction of Prognosis in Clear Cell Renal Cell Carcinoma.Biomed Res Int. 2017;2017:4832931. doi: 10.1155/2017/4832931. Epub 2017 Dec 17. Biomed Res Int. 2017. PMID: 29392135 Free PMC article. Review.
-
miRNA-223 at the crossroads of inflammation and cancer.Cancer Lett. 2019 Jun 1;451:136-141. doi: 10.1016/j.canlet.2019.02.051. Epub 2019 Mar 13. Cancer Lett. 2019. PMID: 30878527 Free PMC article. Review.
Cited by
-
MiR-92b-3p inhibits proliferation and migration of C2C12 cells.Cell Cycle. 2020 Nov;19(21):2906-2917. doi: 10.1080/15384101.2020.1827511. Epub 2020 Oct 12. Cell Cycle. 2020. PMID: 33043788 Free PMC article.
-
Exosomal miR-92b-3p Promotes Chemoresistance of Small Cell Lung Cancer Through the PTEN/AKT Pathway.Front Cell Dev Biol. 2021 May 31;9:661602. doi: 10.3389/fcell.2021.661602. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34136482 Free PMC article.
-
Bone marrow fatty acids affect osteoblastic differentiation through miR-92b-3p in the early stages of postmenopausal osteoporosis.Heliyon. 2023 May 22;9(6):e16513. doi: 10.1016/j.heliyon.2023.e16513. eCollection 2023 Jun. Heliyon. 2023. PMID: 37274695 Free PMC article.
-
Role of TSC1 in physiology and diseases.Mol Cell Biochem. 2021 Jun;476(6):2269-2282. doi: 10.1007/s11010-021-04088-3. Epub 2021 Feb 11. Mol Cell Biochem. 2021. PMID: 33575875 Review.
-
A Review of Recent Research on the Role of MicroRNAs in Renal Cancer.Med Sci Monit. 2021 May 8;27:e930639. doi: 10.12659/MSM.930639. Med Sci Monit. 2021. PMID: 33963171 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

