Synthesis and characterization of bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives
- PMID: 31976009
- PMCID: PMC6964645
- DOI: 10.3762/bjoc.15.296
Synthesis and characterization of bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives
Abstract
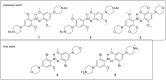
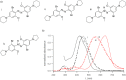
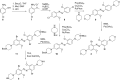
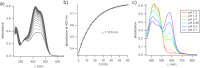
Aminoazobenzene derivatives with four ortho substituents with respect to the N-N double bond are a relatively unexplored class of azo compounds that show promise for use as photoswitches in biology. Tetra-ortho-methoxy-substituted aminoazobenzene compounds in particular can form azonium ions under physiological conditions and exhibit red-light photoswitching. Here, we report the synthesis and characterization of two bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives. These compounds form red-light-absorbing azonium ions, but only under very acidic conditions (pH < 1). While the low pK a makes the azonium form unsuitable, the neutral versions of these compounds undergo trans-to-cis photoisomerization with blue-green light and exhibit slow (τ1/2 ≈ 10 min) thermal reversion and so may find applications under physiological conditions.
Keywords: azobenzene; azonium; molecular switches; ortho substitution; photoisomerization; photoswitch; visible light.
Copyright © 2019, Martínez-López et al.; licensee Beilstein-Institut.
Figures

Similar articles
-
Manipulation of Chemistry and Biology with Visible Light Using Tetra-ortho-Substituted Azobenzenes and Azonium Ions.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202423506. doi: 10.1002/anie.202423506. Epub 2025 Apr 10. Angew Chem Int Ed Engl. 2025. PMID: 40152740 Free PMC article. Review.
-
Red-Shifting Azobenzene Photoswitches for in Vivo Use.Acc Chem Res. 2015 Oct 20;48(10):2662-70. doi: 10.1021/acs.accounts.5b00270. Epub 2015 Sep 28. Acc Chem Res. 2015. PMID: 26415024
-
Mechanistic Basis for Red Light Switching of Azonium Ions.J Am Chem Soc. 2023 Sep 13;145(36):19894-19902. doi: 10.1021/jacs.3c06157. Epub 2023 Sep 1. J Am Chem Soc. 2023. PMID: 37656631
-
Red, far-red, and near infrared photoswitches based on azonium ions.Chem Commun (Camb). 2015 Aug 21;51(65):12981-4. doi: 10.1039/c5cc02804c. Chem Commun (Camb). 2015. PMID: 26176021
-
Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared.Macromol Rapid Commun. 2018 Jan;39(1). doi: 10.1002/marc.201700220. Epub 2017 Jun 23. Macromol Rapid Commun. 2018. PMID: 28643895 Review.
Cited by
-
Manipulation of Chemistry and Biology with Visible Light Using Tetra-ortho-Substituted Azobenzenes and Azonium Ions.Angew Chem Int Ed Engl. 2025 Jun 10;64(24):e202423506. doi: 10.1002/anie.202423506. Epub 2025 Apr 10. Angew Chem Int Ed Engl. 2025. PMID: 40152740 Free PMC article. Review.
-
Molecular photoswitches in aqueous environments.Chem Soc Rev. 2021 Nov 15;50(22):12377-12449. doi: 10.1039/d0cs00547a. Chem Soc Rev. 2021. PMID: 34590636 Free PMC article. Review.
-
Taking phototherapeutics from concept to clinical launch.Nat Rev Chem. 2021 Nov;5(11):816-834. doi: 10.1038/s41570-021-00326-w. Epub 2021 Oct 6. Nat Rev Chem. 2021. PMID: 37117665 Free PMC article. Review.
-
Light-Responsive Solid-Solid Phase Change Materials for Photon and Thermal Energy Storage.ACS Mater Au. 2022 Sep 30;3(1):37-42. doi: 10.1021/acsmaterialsau.2c00055. eCollection 2023 Jan 11. ACS Mater Au. 2022. PMID: 36647455 Free PMC article.
References
-
- García-Iriepa C, Marazzi M, Frutos L M, Sampedro D. RSC Adv. 2013;3(18):6241–6266. doi: 10.1039/c2ra22363e. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
