Synthesis and characterization of bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives
- PMID: 31976009
- PMCID: PMC6964645
- DOI: 10.3762/bjoc.15.296
Synthesis and characterization of bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives
Abstract
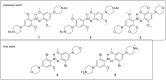
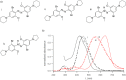
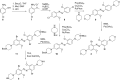
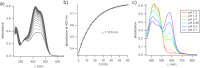
Aminoazobenzene derivatives with four ortho substituents with respect to the N-N double bond are a relatively unexplored class of azo compounds that show promise for use as photoswitches in biology. Tetra-ortho-methoxy-substituted aminoazobenzene compounds in particular can form azonium ions under physiological conditions and exhibit red-light photoswitching. Here, we report the synthesis and characterization of two bis(4-amino-2-bromo-6-methoxy)azobenzene derivatives. These compounds form red-light-absorbing azonium ions, but only under very acidic conditions (pH < 1). While the low pK a makes the azonium form unsuitable, the neutral versions of these compounds undergo trans-to-cis photoisomerization with blue-green light and exhibit slow (τ1/2 ≈ 10 min) thermal reversion and so may find applications under physiological conditions.
Keywords: azobenzene; azonium; molecular switches; ortho substitution; photoisomerization; photoswitch; visible light.
Copyright © 2019, Martínez-López et al.; licensee Beilstein-Institut.
Figures

References
-
- García-Iriepa C, Marazzi M, Frutos L M, Sampedro D. RSC Adv. 2013;3(18):6241–6266. doi: 10.1039/c2ra22363e. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
