Diagnostic Accuracy of a Novel and Rapid Lipoarabinomannan Test for Diagnosing Tuberculosis Among People With Human Immunodeficiency Virus
- PMID: 31976353
- PMCID: PMC6966242
- DOI: 10.1093/ofid/ofz530
Diagnostic Accuracy of a Novel and Rapid Lipoarabinomannan Test for Diagnosing Tuberculosis Among People With Human Immunodeficiency Virus
Abstract
Background: The novel Fujifilm SILVAMP TB-LAM (FujiLAM) assay detects mycobacterial lipoarabinomannan in urine and has demonstrated superior sensitivity to the Alere Determine TB-LAM Ag (AlereLAM) assay for detection of tuberculosis among hospitalized people with human immunodeficiency virus (PWH). This is the first study to evaluate the assay among a broad population referred for antiretroviral therapy including both outpatients (mainly) and inpatients.
Methods: We assessed diagnostic accuracy of FujiLAM and AlereLAM assays in biobanked urine samples from a cohort of adults referred for antiretroviral therapy in Ghana against a microbiological and a composite (including clinical judgement) reference standard, and we assessed the association of FujiLAM test positivity with mortality.
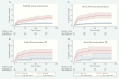
Results: We evaluated urine samples from 532 PWH (462 outpatients, 70 inpatients). Against a microbiological reference standard, the sensitivity of FujiLAM was 74.2% (95% confidence interval [CI], 62.0-84.2) compared to 53.0% (95% CI, 40.3-65.4) for AlereLAM, a difference of 21.2% (CI, 13.1-32.5). Specificity was 89.3% (95% CI, 85.8-92.2) versus 95.6% (95% CI, 93.0-97.4) for FujiLAM and AlereLAM, a difference of -6.3% (95% CI -9.6 to -3.3). Specificity estimates for FujiLAM increased markedly to 98.8% (95% CI, 96.6-99.8) in patients with CD4 >100 cells/µL and when using a composite reference standard. FujiLAM test positivity was associated with increased cumulative risk of mortality at 6 months (hazard ratio, 4.80; 95% CI, 3.01-7.64).
Conclusions: FujiLAM offers significantly increased diagnostic sensitivity in comparison to AlereLAM. Specificity estimates for FujiLAM were lower than for AlereLAM but were affected by the limited ability of the reference standard to correctly diagnose tuberculosis in individuals with low CD4 counts.
Keywords: HIV; LAM; diagnostic accuracy; tuberculosis; urine.
© The Author(s) 2019. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures
References
-
- World Health Organization. Global Tuberculosis Report. Geneva: World Health Organization; 2018.
-
- Pai M, Behr MA, Dowdy D, et al. . Tuberculosis. Nat Rev Dis Primers 2016; 2:16076. - PubMed
-
- World Health Organization. High-Priority Target Product Profiles for New Tuberculosis Diagnostics, Report of a Consenus Meeting. Geneva: World Health Organization; 2014.
-
- World Health Organization. The Use of Lateral Flow Urine Lipoarabinomannan Assay (LF-LAM) for the Diagnosis and Screening of Active Tuberculosis in People Living with HIV. Policy Guidance. Geneva: World Health Organization; 2015.
LinkOut - more resources
Full Text Sources
Research Materials

