Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier
- PMID: 31977002
- PMCID: PMC7099331
- DOI: 10.1182/blood.2019002395
Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier
Erratum in
-
Carpio C, Bouabdallah R, Ysebaert L, et al. Avadomide monotherapy in relapsed/refractory DLBCL: safety, efficacy, and a predictive gene classifier. Blood. 2020;135(13):996-1007.Blood. 2021 Apr 15;137(15):2126. doi: 10.1182/blood.2021011546. Blood. 2021. PMID: 33856449 Free PMC article. No abstract available.
Abstract
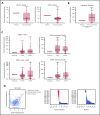
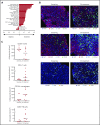
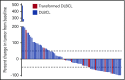
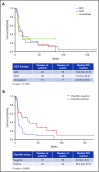
Treatment options for relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL) are limited, with no standard of care; prognosis is poor, with 4- to 6-month median survival. Avadomide (CC-122) is a cereblon-modulating agent with immunomodulatory and direct antitumor activities. This phase 1 dose-expansion study assessed safety and clinical activity of avadomide monotherapy in patients with de novo R/R DLBCL and transformed lymphoma. Additionally, a novel gene expression classifier, which identifies tumors with a high immune cell infiltration, was shown to enrich for response to avadomide in R/R DLBCL. Ninety-seven patients with R/R DLBCL, including 12 patients with transformed lymphoma, received 3 to 5 mg avadomide administered on continuous or intermittent schedules until unacceptable toxicity, disease progression, or withdrawal. Eighty-two patients (85%) experienced ≥1 grade 3/4 treatment-emergent adverse events (AEs), most commonly neutropenia (51%), infections (24%), anemia (12%), and febrile neutropenia (10%). Discontinuations because of AEs occurred in 10% of patients. Introduction of an intermittent 5/7-day schedule improved tolerability and reduced frequency and severity of neutropenia, febrile neutropenia, and infections. Among 84 patients with de novo R/R DLBCL, overall response rate (ORR) was 29%, including 11% complete response (CR). Responses were cell-of-origin independent. Classifier-positive DLBCL patients (de novo) had an ORR of 44%, median progression-free survival (mPFS) of 6 months, and 16% CR vs an ORR of 19%, mPFS of 1.5 months, and 5% CR in classifier-negative patients (P = .0096). Avadomide is being evaluated in combination with other antilymphoma agents. This trial was registered at www.clinicaltrials.gov as #NCT01421524.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: C.C. received travel grants from Takeda, Janssen, Roche, and Celgene, A Bristol-Myers Squibb Company. L.Y. reports research funding from Janssen and Roche, and served on the advisory board for Abbvie, Gilead, Roche, and Janssen. J.-M.S. received travel grants from Roche and honoraria from Roche, Gilead, Janssen, Celgene, A Bristol-Myers Squibb Company, Kern-Pharma and Servier and served on the advisory board of Roche, Gilead, Janssen, Bristol-Myers Squibb, Kern-Pharma, and Celltrion. G.S. received research funding from Roche and Celgene, A Bristol-Myers Squibb Company, and served on the advisory board for Novartis. R.C. participated in a speaker’s bureau for Roche, Janssen, and Celgene, A Bristol-Myers Squibb Company; received honoraria from Roche, Janssen, and Celgene, A Bristol-Myers Squibb Company; served on the advisory board for Janssen, Celgene, A Bristol-Myers Squibb Company, and Servier; and received travel grants from Roche, Janssen, Celgene, A Bristol-Myers Squibb Company, AbbVie, and Pfizer. A.P. participated in a speaker’s bureau for Roche; received patents or royalties from EDO-Mundipharma; received honoraria from Roche, MSD, Bristol-Myers Squibb, and Servier; served on the advisory board of Servier, Roche, Bristol-Myers Squibb, and MSD; and received travel grants from Roche and Takeda. D.R. served on the advisory board of Boehringer Ingelheim and Eli Lilly, received travel grants from Asana, and received research funding from Celgene, A Bristol-Myers Squibb Company. C.P. participated in a speaker’s bureau for Roche and Janssen, served on the advisory board of Bristol-Myers Squibb and Kyowa Kirin, and received travel grants from Roche. J.A.L.-M. participated in a speaker’s bureau for Bristol-Myers Squibb, Roche, and MSD; received patents or royalties and reports equity ownership from PharmaMar; received travel grants from Bristol Myers-Squibb, Roche, and MSD; and received research funding from Iovance, Adaptimmune, Novartis, Roche, Merck, Pfizer, MSD, and Celgene, A Bristol-Myers Squibb Company. A. Santoro participated in a speaker’s bureau for Takeda, Roche, AbbVie, Amgen, Celgene, A Bristol-Myers Squibb Company, AstraZeneca, Eli Lilly, Sandoz, and Novartis and served on the advisory board for Bristol-Myers Squibb, Servier, Gilead, Pfizer, Eisai, Arqule, Bayer, and MSD. A. Salar participated in a speaker’s bureau for Roche and Janssen; received travel grants from Roche; served on the advisory board of Celgene, A Bristol-Myers Squibb Company, Roche, Janssen, Gilead; and reports research funding from Roche and Gilead. S.D. reports research funding from Novartis. A.M. received honoraria from Celgene, A Bristol-Myers Squibb Company, Roche, Janssen, and Servier; served on the advisory board of Roche, Celgene, A Bristol-Myers Squibb Company, and MorPhosys; provided expert testimony on behalf of Gilead; received travel grants from Roche, Celgene, A Bristol-Myers Squibb Company, Janssen, Servier, and Mundipharma; and reports research funding from Celgene, A Bristol-Myers Squibb Company, Janssen, Teva, and Mundipharma. A.W. received patents and royalties from BC Cancer Agency, received travel grants from Dava Oncology, served on the advisory board for m-panels and Guidepoint global, and reports research funding from Celgene, A Bristol-Myers Squibb Company. T.J.B. has equity ownership with Amgen. V.R. received honoraria from Infinity Pharmaceuticals, Bristol-Myers Squibb, Eisai, PharmaMar, and Gilead Sciences; served on the advisory board for Infinity Pharmaceuticals, Bristol-Myers Squibb, PharmaMar, Gilead Sciences, NanoString Technologies, Incyte, MSD, Roche, Genentech, and Epizyme; provided expert testimony on behalf of Servier, reports research funding from arGEN-X BVBA; and received travel grants from Roche and Bristol-Myers Squibb. F.S. has equity ownership with Bristol-Myers Squibb. S. Couto had equity ownership with Celgne, A Bristol-Myers Squibb Company. M.G., S. Carrancio, X. Wei, K.H., M.W.B.T., A.R., M.W., T.J.B., P.R.H., A.K.G., and M.P. are employees of Bristol-Myers Squibb and have equity ownership with Bristol-Myers Squibb. R.B., G.V., E.V.d.N., and X. Wang declare no competing financial interests.
Figures

Similar articles
-
Avadomide plus obinutuzumab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma (CC-122-NHL-001): a multicentre, dose escalation and expansion phase 1 study.Lancet Haematol. 2020 Sep;7(9):e649-e659. doi: 10.1016/S2352-3026(20)30208-8. Epub 2020 Aug 3. Lancet Haematol. 2020. PMID: 32758434 Clinical Trial.
-
Phase I, multicenter, dose-escalation study of avadomide in adult Japanese patients with advanced malignancies.Cancer Sci. 2021 Jan;112(1):331-338. doi: 10.1111/cas.14704. Epub 2020 Nov 26. Cancer Sci. 2021. PMID: 33075165 Free PMC article. Clinical Trial.
-
A First-in-Human Study of Novel Cereblon Modulator Avadomide (CC-122) in Advanced Malignancies.Clin Cancer Res. 2019 Jan 1;25(1):90-98. doi: 10.1158/1078-0432.CCR-18-1203. Epub 2018 Sep 10. Clin Cancer Res. 2019. PMID: 30201761 Clinical Trial.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
-
Efficacy and Safety of Ibrutinib as Monotherapy or Combination Therapy in Relapsed/Refractory Diffuse Large B-cell Lymphoma (R/R DLBCL): A Systematic Review and Meta-analysis.Am J Ther. 2025 Jan-Feb 01;32(1):e5-e16. doi: 10.1097/MJT.0000000000001831. Epub 2024 Oct 16. Am J Ther. 2025. PMID: 39413356 Free PMC article.
Cited by
-
Integrative analysis of hub genes and key pathway in two subtypes of diffuse large B-cell lymphoma by bioinformatics and basic experiments.J Clin Lab Anal. 2021 Nov;35(11):e23978. doi: 10.1002/jcla.23978. Epub 2021 Sep 21. J Clin Lab Anal. 2021. PMID: 34545634 Free PMC article.
-
Avadomide induces degradation of ZMYM2 fusion oncoproteins in hematologic malignancies.Blood Cancer Discov. 2021 May;2(3):250-265. doi: 10.1158/2643-3230.BCD-20-0105. Epub 2021 Mar 10. Blood Cancer Discov. 2021. PMID: 34027417 Free PMC article.
-
Molecular mechanisms of thalidomide and its derivatives.Proc Jpn Acad Ser B Phys Biol Sci. 2020;96(6):189-203. doi: 10.2183/pjab.96.016. Proc Jpn Acad Ser B Phys Biol Sci. 2020. PMID: 32522938 Free PMC article.
-
Immunomodulatory Drugs for the Treatment of B Cell Malignancies.Int J Mol Sci. 2021 Aug 9;22(16):8572. doi: 10.3390/ijms22168572. Int J Mol Sci. 2021. PMID: 34445275 Free PMC article. Review.
-
CD73+CD8+ T cells define a subset with anti-tumor potential in DLBCL patients.Front Med (Lausanne). 2025 May 2;12:1526772. doi: 10.3389/fmed.2025.1526772. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40385579 Free PMC article.
References
-
- Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin. 2016;66(6):443-459. - PubMed
-
- Sant M, Allemani C, Tereanu C, et al. ; HAEMACARE Working Group . Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724-3734. - PubMed
-
- Philip T, Guglielmi C, Hagenbeek A, et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med. 1995;333(23):1540-1545. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

