Xeno-Free Reprogramming of Peripheral Blood Mononuclear Erythroblasts on Laminin-521
- PMID: 31977148
- PMCID: PMC7176073
- DOI: 10.1002/cpsc.103
Xeno-Free Reprogramming of Peripheral Blood Mononuclear Erythroblasts on Laminin-521
Abstract
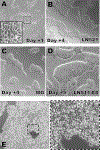
Translating human induced pluripotent stem cell (hiPSC)-derived cells and tissues into the clinic requires streamlined and reliable production of clinical-grade hiPSCs. This article describes an entirely animal component-free procedure for the reliable derivation of stable hiPSC lines from donor peripheral blood mononuclear cells (PBMCs) using only autologous patient materials and xeno-free reagents. PBMCs are isolated from a whole blood donation, from which a small amount of patient serum is also generated. The PBMCs are then expanded prior to reprogramming in an animal component-free erythroblast growth medium supplemented with autologous patient serum, thereby eliminating the need for animal serum. After expansion, the erythroblasts are reprogrammed using either cGMP-grade Sendai viral particles (CytoTune™ 2.1 kit) or episomally replicating reprogramming plasmids (Epi5™ kit), both commercially available. Expansion of emerging hiPSCs on a recombinant cGMP-grade human laminin substrate is compatible with a number of xeno-free or chemically defined media (some available as cGMP-grade reagents), such as E8, Nutristem, Stemfit, or mTeSR Plus. hiPSC lines derived using this method display expression of expected surface markers and transcription factors, loss of the reprogramming agent-derived nucleic acids, genetic stability, and the ability to robustly differentiate in vitro to multiple lineages. © 2020 by John Wiley & Sons, Inc. Basic Protocol 1: Isolating peripheral blood mononuclear cells using CPT tubes Support Protocol 1: Removal of clotting factors to produce serum from autologous plasma collected in Basic Protocol 1 Basic Protocol 2: PBMC expansion in an animal-free erythroblast expansion medium containing autologous serum Basic Protocol 3: Reprogramming of expanded PBMCs with Sendai viral reprogramming particles Alternate Protocol: Reprogramming of expanded PBMCs with episomal plasmids Basic Protocol 4: Picking, expanding, and cryopreserving hiPSC clones Support Protocol 2: Testing Sendai virus kit-reprogrammed hiPSC for absence of Sendai viral RNA Support Protocol 3: Testing Epi5 kit-reprogrammed hiPSC for absence of episomal plasmid DNA Support Protocol 4: Assessing the undifferentiated state of human pluripotent stem cell cultures by multi-color immunofluorescent staining and confocal imaging Support Protocol 5: Coating plates with extracellular matrices to support hiPSC attachment and expansion.
Keywords: Sendai viral reprogramming; episomal reprogramming; human induced pluripotent stem cells (hiPSCs); reprogramming.
© 2020 John Wiley & Sons, Inc.
Figures



Similar articles
-
Human Induced Pluripotent Stem Cell Production and Expansion from Blood using a Non-Integrating Viral Reprogramming Vector.Curr Protoc Mol Biol. 2018 Apr;122(1):e58. doi: 10.1002/cpmb.58. Curr Protoc Mol Biol. 2018. PMID: 29851250 Free PMC article.
-
Generation of human iPSCs from human peripheral blood mononuclear cells using non-integrative Sendai virus in chemically defined conditions.Methods Mol Biol. 2013;1036:81-8. doi: 10.1007/978-1-62703-511-8_7. Methods Mol Biol. 2013. PMID: 23807788 Free PMC article.
-
Generation of clinical-grade human induced pluripotent stem cells in Xeno-free conditions.Stem Cell Res Ther. 2015 Nov 12;6:223. doi: 10.1186/s13287-015-0206-y. Stem Cell Res Ther. 2015. PMID: 26564165 Free PMC article.
-
Nonintegrating Human Somatic Cell Reprogramming Methods.Adv Biochem Eng Biotechnol. 2018;163:1-21. doi: 10.1007/10_2017_29. Adv Biochem Eng Biotechnol. 2018. PMID: 29075799 Review.
-
Cellular reprogramming of human peripheral blood cells.Genomics Proteomics Bioinformatics. 2013 Oct;11(5):264-74. doi: 10.1016/j.gpb.2013.09.001. Epub 2013 Sep 21. Genomics Proteomics Bioinformatics. 2013. PMID: 24060839 Free PMC article. Review.
Cited by
-
The Low Tumorigenic Risk and Subtypes of Cardiomyocytes Derived from Human-induced Pluripotent Stem Cells.Curr Stem Cell Res Ther. 2025;20(3):317-335. doi: 10.2174/011574888X318139240621051224. Curr Stem Cell Res Ther. 2025. PMID: 40351082
-
An engineered Sox17 induces somatic to neural stem cell fate transitions independently from pluripotency reprogramming.Sci Adv. 2023 Aug 25;9(34):eadh2501. doi: 10.1126/sciadv.adh2501. Epub 2023 Aug 23. Sci Adv. 2023. PMID: 37611093 Free PMC article.
-
Induced Pluripotent Stem Cells as a Tool for Modeling Hematologic Disorders and as a Potential Source for Cell-Based Therapies.Cells. 2021 Nov 19;10(11):3250. doi: 10.3390/cells10113250. Cells. 2021. PMID: 34831472 Free PMC article. Review.
References
-
- Ban H, Nishishita N, Fusaki N, Tabata T, Saeki K, Shikamura M, … Nishikawa S (2011). Efficient generation of transgene-free human induced pluripotent stem cells (iPSCs) by temperature-sensitive Sendai virus vectors. Proc Natl Acad Sci U S A, 108(34), 14234–14239. doi:10.1073/pnas.1103509108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

