Identification of Psychoplastogenic N, N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure-Activity Relationship Studies
- PMID: 31977208
- PMCID: PMC7075704
- DOI: 10.1021/acs.jmedchem.9b01404
Identification of Psychoplastogenic N, N-Dimethylaminoisotryptamine (isoDMT) Analogues through Structure-Activity Relationship Studies
Abstract
Ketamine, N,N-dimethyltryptamine (DMT), and other psychoplastogens possess enormous potential as neurotherapeutics due to their ability to potently promote neuronal growth. Here, we report the first-ever structure-activity relationship study with the explicit goal of identifying novel psychoplastogens. We have discovered several key features of the psychoplastogenic pharmacophore and used this information to develop N,N-dimethylaminoisotryptamine (isoDMT) psychoplastogens that are easier to synthesize, have improved physicochemical properties, and possess reduced hallucinogenic potential as compared to their DMT counterparts.
Figures
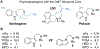

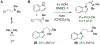


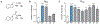

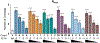


References
-
- Whiteford HA; Degenhardt L; Rehm J; Baxter AJ; Ferrari AJ; Erskine HE; Charlson FJ; Norman RE; Flaxman AD; Johns N, Murray CJL; Vos Theo. Global Burden of Disease Attributable to Mental and Substance Use Disorders: Findings from the Global Burden of Disease Study. Lancet 2013, 382, 1575–1586. - PubMed
-
- Moda-Sava RN; Murdock MH; Parekh PK; Fetcho RN; Huang BS; Huynh TN; Witztum J; Shaver DC; Rosenthal DL; Alway EJ; Lopez K; Meng Y; Nellissen L; Grosenick L; Milner TA; Deisseroth K; Bito H; Kasai H; Liston C6. Sustained Rescue of Prefrontal Circuit Dysfunction by Antidepressant-Induced Spine Formation. Science 2019, 364 DOI: 10.1126/science.aat8078 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

