Annulation of Hydrazones and Alkynes via Rhodium(III)-Catalyzed Dual C-H Activation: Synthesis of Pyrrolopyridazines and Azolopyridazines
- PMID: 31977232
- PMCID: PMC7007857
- DOI: 10.1021/acs.orglett.0c00186
Annulation of Hydrazones and Alkynes via Rhodium(III)-Catalyzed Dual C-H Activation: Synthesis of Pyrrolopyridazines and Azolopyridazines
Abstract
Hydrazones readily synthesized from N-aminopyrroles or N-aminoazoles and aldehydes undergo Rh(III)-catalyzed dual C-H activation and coupling with aryl- and alkyl-substituted alkynes to give pyrrolopyridazines or azolopyridazines, respectively. This transformation represents a rare example of hydrazoyl C-H activation and proceeds without heteroatom functionality to direct C-H activation. Hydrazones derived from aromatic, alkenyl, and aliphatic aldehydes were effective inputs, and tethering the alkyne to the hydrazone enabled annulations to more complex, tricyclic products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



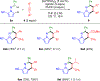



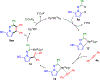
References
-
- Halskov KS; Witten MR; Hoang GL; Mercado BQ; Ellman JA Rhodium(III)-Catalyzed Imidoyl C–H Activation for Annulations to Azolopyrimidines. Org. Lett 2018, 20, 2464–2467. - PMC - PubMed
- Hoang GL; Li Y; Halskov KS; Ellman JA Synthesis of Azolo[1,3,5]triazines via Rhodium(III)-Catalyzed Annulation of N-Azolo Imines and Dioxazolones. J. Org. Chem 2018, 83, 9522–9529. - PMC - PubMed
- Hoang GL; Streit AD; Ellman JA Three-Component Coupling of Aldehydes, Aminopyrazoles, and Sulfoxonium Ylides via Rhodium(III)-Catalyzed Imidoyl C–H Activation: Synthesis of Pyrazolo[1,5-a]pyrimidines. J. Org. Chem 2018, 83, 15347. - PMC - PubMed
- Zhang Y; Zhu H; Huang Y; Hu Q; He Y; Wen Y; Zhu G Multicomponent Synthesis of Isoindolinones by RhIII Relay Catalysis: Synthesis of Pagoclone and Pazinaclone from Benzaldehyde. Org. Lett 2019, 21, 1273–1277. - PubMed
- Hoang GL; Zoll AJ; Ellman JA Three-Component Coupling of Aldehydes, 2-Aminopyridines, and Diazo Esters via Rhodium(III)-Catalyzed Imidoyl C–H Activation: Synthesis of Pyrido[1,2-a]pyrimidin-4-ones. Org. Lett 2019, 21, 3886–3890. - PMC - PubMed
-
- For reviews, see: Oukoloff K; Lucero B; Francisco KR; Brunden KR; Ballatore C 1,2,4-Triazolo[1,5-a]pyrimidines in Drug Design. Eur. J. Med. Chem 2019, 165, 332–346. - PMC - PubMed
- Cherukupalli S; Karpoormath R; Chandrasekaran B; Hampannavar GA; Thapliyal N; Palakollu VN An Insight on Synthetic and Medicinal Aspects of Pyrazolo[1,5-a]pyrimidine Scaffold. Eur. J. Med. Chem 2017, 126, 298–352. - PubMed
- Bagdi AK; Santra S; Monir K; Hajra A Synthesis of Imidazo[1,2-a]pyridines: A Decade Update. Chem. Commun 2015, 51, 1555–1575. - PubMed
-
- Hayashi Y; Satoh T; Miura M; Kawauchi S Theoretical Investigation of Regioselectivity in the Rh-Catalyzed Coupling Reaction of 3-Phenylthiophene with Styrene. Eur. J. Org. Chem 2019, 2019, 2998–3004.
- Iitsuka T; Hirano K; Satoh T; Miura M Rhodium-Catalyzed Dehydrogenative Coupling of Phenylheteroarenes with Alkynes or Alkenes. J. Org Chem 2015, 80, 2804–2814. - PubMed
- Iitsuka T; Hirano K; Satoh T; Miura M Rhodium-Catalyzed Annulative Coupling of 3-Phenylthiophenes with Alkynes Involving Double C–H Bond Cleavages. Chem. Eur. J 2014, 20, 385–389. - PubMed
- Huang J-R; Zhang Q-R; Qu C-H; Sun X-H; Dong L; Chen Y-C Rhodium(III)-Catalyzed Direct Selective C(5)−H Oxidative Annulations of 2-Substituted Imidazoles and Alkynes by Double C−H Activation. Org. Lett 2013, 15, 1878–1881. - PubMed
-
- For a review on dual C-H activation with alkyne coupling, see: Li S-S; Qin L; Dong L Rhodium-Catalyzed C–C Coupling Reactions via Double C–H Activation. Org. Biomol. Chem 2016, 14, 4554–4570. - PubMed
-
- Ge Q; Hu Y; Li B; Wang B Synthesis of Conjugated Polycyclic Quinoliniums by Rhodium(III)-Catalyzed Multiple C–H Activation and Annulation of Arylpyridiniums with Alkynes. Org. Lett 2016, 18, 2483–2486. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

