Pulsed Field Ablation Versus Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model
- PMID: 31977250
- PMCID: PMC7069397
- DOI: 10.1161/CIRCEP.119.008303
Pulsed Field Ablation Versus Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model
Abstract
Background: Pulsed field ablation (PFA) can be myocardium selective, potentially sparing the esophagus during left atrial ablation. In an in vivo porcine esophageal injury model, we compared the effects of newer biphasic PFA with radiofrequency ablation (RFA).
Methods: In 10 animals, under general anesthesia, the lower esophagus was deflected toward the inferior vena cava using an esophageal deviation balloon, and ablation was performed from within the inferior vena cava at areas of esophageal contact. Four discrete esophageal sites were targeted in each animal: 6 animals received 8 PFA applications/site (2 kV, multispline catheter), and 4 animals received 6 clusters of irrigated RFA applications (30 W×30 seconds, 3.5 mm catheter). All animals were survived to 25 days, sacrificed, and the esophagus submitted for pathological examination, including 10 discrete histological sections/esophagus.
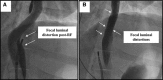
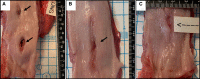
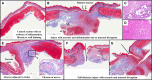
Results: The animals weight increased by 13.7±6.2% and 6.8±6.3% (P=0.343) in the PFA and RFA cohorts, respectively. No PFA animals (0 of 6, 0%) developed abnormal in-life observations, but 1 of 4 RFA animals (25%) developed fever and dyspnea. On necropsy, no PFA animals (0 of 6, 0%) demonstrated esophageal lesions. In contrast, esophageal injury occurred in all RFA animals (4 of 4, 100%; P=0.005): a mean of 1.5 mucosal lesions/animal (length, -21.8±8.9 mm; width, -4.9±1.4 mm) were observed, including one esophago-pulmonary fistula and deep esophageal ulcers in the other animals. Histological examination demonstrated tissue necrosis surrounded by acute and chronic inflammation and fibrosis. The necrotic RFA lesions involved multiple esophageal tissue layers with evidence of arteriolar medial thickening and fibrosis of periesophageal nerves. Abscess formation and full-thickness esophageal wall disruptions were seen in areas of perforation/fistula.
Conclusions: In this novel porcine model of esophageal injury, biphasic PFA induced no chronic histopathologic esophageal changes, while RFA demonstrated a spectrum of esophageal lesions including fistula and deep esophageal ulcers and abscesses.
Keywords: atrial fibrillation; catheter ablation; electroporation; fistula; swine.
Figures





References
-
- Koruth JS, Chu EW, Bhardwaj R, Dukkipati S, Reddy VY. Esophageal damage during epicardial ventricular tachycardia ablation. JACC Clin Electrophysiol. 2017;3:1470–1471. doi: 10.1016/j.jacep.2017.03.016. - PubMed
-
- Kapur S, Barbhaiya C, Deneke T, Michaud GF. Esophageal injury and atrioesophageal fistula caused by ablation for atrial fibrillation. Circulation. 2017;136:1247–1255. doi: 10.1161/CIRCULATIONAHA.117.025827. - PubMed
-
- Lim HW, Cogert GA, Cameron CS, Cheng VY, Sandler DA. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2014;25:208–213. doi: 10.1111/jce.12313. - PubMed
-
- Black-Maier E, Pokorney SD, Barnett AS, Zeitler EP, Sun AY, Jackson KP, Bahnson TD, Daubert JP, Piccini JP. Risk of atrioesophageal fistula formation with contact force-sensing catheters. Heart Rhythm. 2017;14:1328–1333. doi: 10.1016/j.hrthm.2017.04.024. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

