Temperature-Sensitive Frozen-Tissue Imaging for Cryoablation Monitoring Using STIR-UTE MRI
- PMID: 31977600
- PMCID: PMC7145748
- DOI: 10.1097/RLI.0000000000000642
Temperature-Sensitive Frozen-Tissue Imaging for Cryoablation Monitoring Using STIR-UTE MRI
Abstract
Purpose: The aim of this study was to develop a method to delineate the lethally frozen-tissue region (temperature less than -40°C) arising from interventional cryoablation procedures using a short tau inversion-recovery ultrashort echo-time (STIR-UTE) magnetic resonance (MR) imaging sequence. This method could serve as an intraprocedural validation of the completion of tumor ablation, reducing the number of local recurrences after cryoablation procedures.
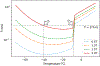
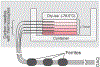
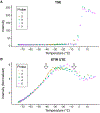
Materials and methods: The method relies on the short T1 and T2* relaxation times of frozen soft tissue. Pointwise Encoding Time with Radial Acquisition, a 3-dimensional UTE sequence with TE = 70 microseconds, was optimized with STIR to null tissues with a T1 of approximately 271 milliseconds, the threshold T1. Because the T1 relaxation time of frozen tissue in the temperature range of -40°C < temperature < -8°C is shorter than the threshold T1 at the 3-tesla magnetic field, tissues in this range should appear hyperintense. The sequence was evaluated in ex vivo frozen tissue, where image intensity and actual tissue temperatures, measured by thermocouples, were correlated. Thereafter, the sequence was evaluated clinically in 12 MR-guided prostate cancer cryoablations, where MR-compatible cryoprobes were used to destroy cancerous tissue and preserve surrounding normal tissue.
Results: The ex vivo experiment using a bovine muscle demonstrated that STIR-UTE images showed regions approximately between -40°C and -8°C as hyperintense, with tissues at lower and higher temperatures appearing dark, making it possible to identify the region likely to be above the lethal temperature inside the frozen tissue. In the clinical cases, the STIR-UTE images showed a dark volume centered on the cryoprobe shaft, Vinner, where the temperature is likely below -40°C, surrounded by a doughnut-shaped hyperintense volume, where the temperature is likely between -40°C and -8°C. The hyperintense region was itself surrounded by a dark volume, where the temperature is likely above -8°C, permitting calculation of Vouter. The STIR-UTE frozen-tissue volumes, Vinner and Vouter, appeared significantly smaller than signal voids on turbo spin echo images (P < 1.0 × 10), which are currently used to quantify the frozen-tissue volume ("the iceball"). The ratios of the Vinner and Vouter volumes to the iceball were 0.92 ± 0.08 and 0.29 ± 0.07, respectively. In a single postablation follow-up case, a strong correlation was seen between Vinner and the necrotic volume.
Conclusions: Short tau inversion-recovery ultrashort echo-time MR imaging successfully delineated the area approximately between -40°C and -8°C isotherms in the frozen tissue, demonstrating its potential to monitor the lethal ablation volume during MR-guided cryoablation.
Conflict of interest statement
Conflicts of Interest
The PETRA sequence was provided by Siemens Healthineers as a work-in-progress (WIP) package. JT has received research funding from Siemens Healthineers for an unrelated study.
Figures
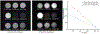



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J. Clin 2019;69(1):7–34. - PubMed
-
- Gandaglia G, Albers P, Abrahamsson P-A, et al. Structured Population-based Prostate-specific Antigen Screening for Prostate Cancer: The European Association of Urology Position in 2019. Eur. Urol 2019;76(2):142–150. - PubMed
-
- Fenton JJ, Weyrich MS, Durbin S, et al. Prostate-Specific Antigen-Based Screening for Prostate Cancer: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2018;319(18):1914–1931. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

