A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures
- PMID: 31977817
- PMCID: PMC7508283
- DOI: 10.2106/JBJS.19.00790
A Randomized, Placebo-Controlled Study of Romosozumab for the Treatment of Hip Fractures
Abstract
Background: Romosozumab is a bone-forming antibody that increases bone formation and decreases bone resorption. We conducted a double-blinded, randomized, phase-2, dose-finding trial to evaluate the effect of romosozumab on the clinical outcomes of open reduction and internal fixation of intertrochanteric or femoral neck hip fractures.
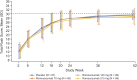
Methods: Patients (55 to 94 years old) were randomized 2:3:3:3 to receive 3 subcutaneous injections of romosozumab (70, 140, or 210 mg) or a placebo postoperatively on day 1 and weeks 2, 6, and 12. The primary end point was the difference in the mean timed "Up & Go" (TUG) score over weeks 6 to 20 for romosozumab versus placebo. Additional end points included the time to radiographic evidence of healing and the score on the Radiographic Union Scale for Hip (RUSH).
Results: A total of 332 patients were randomized: 243 to receive romosozumab (70 mg, n = 60; 140 mg, n = 93; and 210 mg, n = 90) and 89 to receive a placebo. Although TUG scores improved during the study, they did not differ significantly between the romosozumab and placebo groups over weeks 6 to 20 (p = 0.198). The median time to radiographic evidence of healing was 16.4 to 16.9 weeks across treatment groups. The RUSH scores improved over time across treatment groups but did not differ significantly between the romosozumab and placebo groups. The overall safety and tolerability profile of romosozumab was comparable with that of the placebo.
Conclusions: Romosozumab did not improve the fracture-healing-related clinical and radiographic outcomes in the study population.
Level of evidence: Therapeutic Level I. See Instructions for Authors for a complete description of levels of evidence.
Figures


References
-
- Odén A, McCloskey EV, Johansson H, Kanis JA. Assessing the impact of osteoporosis on the burden of hip fractures. Calcif Tissue Int. 2013. January;92(1):42-9. Epub 2012 Nov 8. - PubMed
-
- Johnell O, Kanis JA. An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int. 2004. November;15(11):897-902. Epub 2004 May 4. - PubMed
-
- Bentler SE, Liu L, Obrizan M, Cook EA, Wright KB, Geweke JF, Chrischilles EA, Pavlik CE, Wallace RB, Ohsfeldt RL, Jones MP, Rosenthal GE, Wolinsky FD. The aftermath of hip fracture: discharge placement, functional status change, and mortality. Am J Epidemiol. 2009. November 15;170(10):1290-9. Epub 2009 Oct 4. - PMC - PubMed
-
- International Osteoporosis Foundation. Facts and statistics.Accessed2019Sep13. Accessed 2019 Sep 13 https://www.iofbonehealth.org/facts-statistics#category-16
-
- Kanakaris NK, West RM, Giannoudis PV. Enhancement of hip fracture healing in the elderly: evidence deriving from a pilot randomized trial. Injury. 2015. August;46(8):1425-8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

