Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study
- PMID: 31978055
- PMCID: PMC6980402
- DOI: 10.1371/journal.pmed.1003012
Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study
Erratum in
-
Correction: Dysregulation of multiple metabolic networks related to brain transmethylation and polyamine pathways in Alzheimer disease: A targeted metabolomic and transcriptomic study.PLoS Med. 2020 Oct 21;17(10):e1003439. doi: 10.1371/journal.pmed.1003439. eCollection 2020 Oct. PLoS Med. 2020. PMID: 33085665 Free PMC article.
Abstract
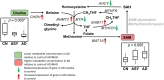
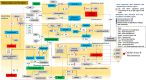
Background: There is growing evidence that Alzheimer disease (AD) is a pervasive metabolic disorder with dysregulation in multiple biochemical pathways underlying its pathogenesis. Understanding how perturbations in metabolism are related to AD is critical to identifying novel targets for disease-modifying therapies. In this study, we test whether AD pathogenesis is associated with dysregulation in brain transmethylation and polyamine pathways.
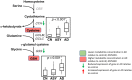
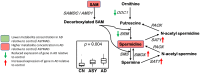
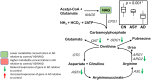
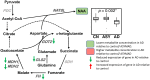
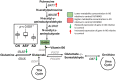
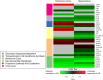
Methods and findings: We first performed targeted and quantitative metabolomics assays using capillary electrophoresis-mass spectrometry (CE-MS) on brain samples from three groups in the Baltimore Longitudinal Study of Aging (BLSA) (AD: n = 17; Asymptomatic AD [ASY]: n = 13; Control [CN]: n = 13) (overall 37.2% female; mean age at death 86.118 ± 9.842 years) in regions both vulnerable and resistant to AD pathology. Using linear mixed-effects models within two primary brain regions (inferior temporal gyrus [ITG] and middle frontal gyrus [MFG]), we tested associations between brain tissue concentrations of 26 metabolites and the following primary outcomes: group differences, Consortium to Establish a Registry for Alzheimer's Disease (CERAD) (neuritic plaque burden), and Braak (neurofibrillary pathology) scores. We found significant alterations in concentrations of metabolites in AD relative to CN samples, as well as associations with severity of both CERAD and Braak, mainly in the ITG. These metabolites represented biochemical reactions in the (1) methionine cycle (choline: lower in AD, p = 0.003; S-adenosyl methionine: higher in AD, p = 0.005); (2) transsulfuration and glutathione synthesis (cysteine: higher in AD, p < 0.001; reduced glutathione [GSH]: higher in AD, p < 0.001); (3) polyamine synthesis/catabolism (spermidine: higher in AD, p = 0.004); (4) urea cycle (N-acetyl glutamate: lower in AD, p < 0.001); (5) glutamate-aspartate metabolism (N-acetyl aspartate: lower in AD, p = 0.002); and (6) neurotransmitter metabolism (gamma-amino-butyric acid: lower in AD, p < 0.001). Utilizing three Gene Expression Omnibus (GEO) datasets, we then examined mRNA expression levels of 71 genes encoding enzymes regulating key reactions within these pathways in the entorhinal cortex (ERC; AD: n = 25; CN: n = 52) and hippocampus (AD: n = 29; CN: n = 56). Complementing our metabolomics results, our transcriptomics analyses also revealed significant alterations in gene expression levels of key enzymatic regulators of biochemical reactions linked to transmethylation and polyamine metabolism. Our study has limitations: our metabolomics assays measured only a small proportion of all metabolites participating in the pathways we examined. Our study is also cross-sectional, limiting our ability to directly test how AD progression may impact changes in metabolite concentrations or differential-gene expression. Additionally, the relatively small number of brain tissue samples may have limited our power to detect alterations in all pathway-specific metabolites and their genetic regulators.
Conclusions: In this study, we observed broad dysregulation of transmethylation and polyamine synthesis/catabolism, including abnormalities in neurotransmitter signaling, urea cycle, aspartate-glutamate metabolism, and glutathione synthesis. Our results implicate alterations in cellular methylation potential and increased flux in the transmethylation pathways, increased demand on antioxidant defense mechanisms, perturbations in intermediate metabolism in the urea cycle and aspartate-glutamate pathways disrupting mitochondrial bioenergetics, increased polyamine biosynthesis and breakdown, as well as abnormalities in neurotransmitter metabolism that are related to AD.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Xu J, Begley P, Church SJ, Patassini S, Hollywood KA, Jullig M, et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim Biophys Acta. 2016;1862(6):1084–92. Epub 2016/03/10. 10.1016/j.bbadis.2016.03.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

