Early peripheral clearance of leukemia-associated immunophenotypes in AML: centralized analysis of a randomized trial
- PMID: 31978214
- PMCID: PMC6988394
- DOI: 10.1182/bloodadvances.2019000406
Early peripheral clearance of leukemia-associated immunophenotypes in AML: centralized analysis of a randomized trial
Abstract
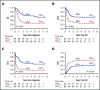
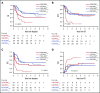
Although genetics is a relevant risk factor in acute myeloid leukemia (AML), it can be minimally informative and/or not readily available for the early identification of patients at risk for treatment failure. In a randomized trial comparing standard vs high-dose induction (ClinicalTrials.gov #NCT00495287), we studied early peripheral blast cell clearance (PBC) as a rapid predictive assay of chemotherapy response to determine whether it correlates with the achievement of complete remission (CR), as well as postremission outcome, according to induction intensity. Individual leukemia-associated immunophenotypes (LAIPs) identified pretherapy by flow cytometry were validated and quantified centrally after 3 days of treatment, expressing PBC on a logarithmic scale as the ratio of absolute LAIP+ cells on day 1 and day 4. Of 178 patients, 151 (84.8%) were evaluable. Patients in CR exhibited significantly higher median PBC (2.3 log) compared with chemoresistant patients (1.0 log; P < .0001). PBC < 1.0 predicted the worst outcome (CR, 28%). With 1.5 log established as the most accurate cutoff predicting CR, 87.5% of patients with PBC >1.5 (PBChigh, n = 96) and 43.6% of patients with PBC ≤1.5 (PBClow, n = 55) achieved CR after single-course induction (P < .0001). CR and PBChigh rates were increased in patients randomized to the high-dose induction arm (P = .04) and correlated strongly with genetic/cytogenetic risk. In multivariate analysis, PBC retained significant predictive power for CR, relapse risk, and survival. Thus, PBC analysis can provide a very early prediction of outcome, correlates with treatment intensity and disease subset, and may support studies of customized AML therapy.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures


References
-
- Döhner H, Estey EH, Amadori S, et al. ; European LeukemiaNet . Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453-474. - PubMed
-
- Röllig C, Bornhäuser M, Thiede C, et al. . Long-term prognosis of acute myeloid leukemia according to the new genetic risk classification of the European LeukemiaNet recommendations: evaluation of the proposed reporting system. J Clin Oncol. 2011;29(20):2758-2765. - PubMed
-
- San Miguel JF, Martínez A, Macedo A, et al. . Immunophenotyping investigation of minimal residual disease is a useful approach for predicting relapse in acute myeloid leukemia patients. Blood. 1997;90(6):2465-2470. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

