Wheat germ agglutinin-conjugated fluorescent pH sensors for visualizing proton fluxes
- PMID: 31978216
- PMCID: PMC7266149
- DOI: 10.1085/jgp.201912498
Wheat germ agglutinin-conjugated fluorescent pH sensors for visualizing proton fluxes
Abstract
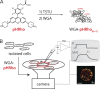
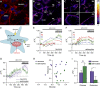
Small-molecule fluorescent wheat germ agglutinin (WGA) conjugates are routinely used to demarcate mammalian plasma membranes, because they bind to the cell's glycocalyx. Here, we describe the derivatization of WGA with a pH-sensitive rhodamine fluorophore (pHRho; pKa = 7) to detect proton channel fluxes and extracellular proton accumulation and depletion from primary cells. We found that WGA-pHRho labeling was uniform and did not appreciably alter the voltage gating of glycosylated ion channels, and the extracellular changes in pH correlated with proton channel activity. Using single-plane illumination techniques, WGA-pHRho was used to detect spatiotemporal differences in proton accumulation and depletion over the extracellular surface of cardiomyocytes, astrocytes, and neurons. Because WGA can be derivatized with any small-molecule fluorescent ion sensor, WGA conjugates should prove useful to visualize most electrogenic and nonelectrogenic events on the extracellular side of the plasma membrane.
© 2020 Zhang et al.
Figures





Similar articles
-
Fluorescent Visualization of Cellular Proton Fluxes.Cell Chem Biol. 2016 Dec 22;23(12):1449-1457. doi: 10.1016/j.chembiol.2016.10.013. Epub 2016 Dec 1. Cell Chem Biol. 2016. PMID: 27916567 Free PMC article.
-
Succinylated Wheat Germ Agglutinin Colocalizes with the Toxoplasma gondii Cyst Wall Glycoprotein CST1.mSphere. 2020 Mar 4;5(2):e00031-20. doi: 10.1128/mSphere.00031-20. mSphere. 2020. PMID: 32132158 Free PMC article.
-
Effect of wheat germ agglutinin density on cellular uptake and toxicity of wheat germ agglutinin conjugated PEG-PLA nanoparticles in Calu-3 cells.Int J Pharm. 2011 Jul 15;413(1-2):184-93. doi: 10.1016/j.ijpharm.2011.04.026. Epub 2011 Apr 28. Int J Pharm. 2011. PMID: 21550388
-
Subunit unbinding mechanics of dimeric wheat germ agglutinin (WGA) studied by atomic force microscopy.FEBS Lett. 2014 Nov 28;588(23):4472-7. doi: 10.1016/j.febslet.2014.10.018. FEBS Lett. 2014. PMID: 25448988
-
WGA-QD probe-based AFM detects WGA-binding sites on cell surface and WGA-induced rigidity alternation.Biochem Biophys Res Commun. 2009 Feb 6;379(2):335-40. doi: 10.1016/j.bbrc.2008.12.058. Epub 2008 Dec 25. Biochem Biophys Res Commun. 2009. PMID: 19103166
Cited by
-
Shedding light on the cell biology and diverse physiological functions of the migrasome.Cell Biosci. 2025 May 28;15(1):70. doi: 10.1186/s13578-025-01417-7. Cell Biosci. 2025. PMID: 40437622 Free PMC article. Review.
-
Unveiling cellular communications through rapid pan-membrane-protein labeling.Nat Commun. 2025 Apr 15;16(1):3584. doi: 10.1038/s41467-025-58779-2. Nat Commun. 2025. PMID: 40234465 Free PMC article.
-
Optical sorting: past, present and future.Light Sci Appl. 2025 Feb 27;14(1):103. doi: 10.1038/s41377-024-01734-5. Light Sci Appl. 2025. PMID: 40011460 Free PMC article. Review.
References
-
- Aras M.A., Hartnett K.A., and Aizenman E.. 2008. Assessment of cell viability in primary neuronal cultures. Curr. Protoc. Neurosci. Chapter 7:Unit 7.18. - PubMed
-
- Colecraft H.M., Alseikhan B., Takahashi S.X., Chaudhuri D., Mittman S., Yegnasubramanian V., Alvania R.S., Johns D.C., Marbán E., and Yue D.T.. 2002. Novel functional properties of Ca(2+) channel beta subunits revealed by their expression in adult rat heart cells. J. Physiol. 541:435–452. 10.1113/jphysiol.2002.018515 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

