Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: a network meta-analysis
- PMID: 31978256
- PMCID: PMC6984637
- DOI: 10.1002/14651858.CD013125.pub2
Antibiotic prophylaxis to prevent spontaneous bacterial peritonitis in people with liver cirrhosis: a network meta-analysis
Abstract
Background: Approximately 2.5% of all hospitalisations in people with liver cirrhosis are for spontaneous bacterial peritonitis. Spontaneous bacterial peritonitis is associated with significant short-term mortality; therefore, it is important to prevent spontaneous bacterial peritonitis in people at high risk of developing it. Antibiotic prophylaxis forms the mainstay preventive method, but this has to be balanced against the development of drug-resistant spontaneous bacterial peritonitis, which is difficult to treat, and other adverse events. Several different prophylactic antibiotic treatments are available; however, there is uncertainty surrounding their relative efficacy and optimal combination.
Objectives: To compare the benefits and harms of different prophylactic antibiotic treatments for prevention of spontaneous bacterial peritonitis in people with liver cirrhosis using a network meta-analysis and to generate rankings of the different prophylactic antibiotic treatments according to their safety and efficacy.
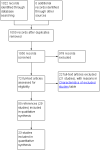
Search methods: We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, World Health Organization International Clinical Trials Registry Platform, and trials registers to November 2018 to identify randomised clinical trials in people with cirrhosis at risk of developing spontaneous bacterial peritonitis.
Selection criteria: We included only randomised clinical trials (irrespective of language, blinding, or status) in adults with cirrhosis undergoing prophylactic treatment to prevent spontaneous bacterial peritonitis. We excluded randomised clinical trials in which participants had previously undergone liver transplantation, or were receiving antibiotics for treatment of spontaneous bacterial peritonitis or other purposes.
Data collection and analysis: We performed a network meta-analysis with OpenBUGS using Bayesian methods and calculated the odds ratio, rate ratio, and hazard ratio (HR) with 95% credible intervals (CrI) based on an available-case analysis, according to National Institute of Health and Care Excellence Decision Support Unit guidance.
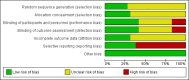
Main results: We included 29 randomised clinical trials (3896 participants; nine antibiotic regimens (ciprofloxacin, neomycin, norfloxacin, norfloxacin plus neomycin, norfloxacin plus rifaximin, rifaximin, rufloxacin, sparfloxacin, sulfamethoxazole plus trimethoprim), and 'no active intervention' in the review. Twenty-three trials (2587 participants) were included in one or more outcomes in the review. The trials that provided the information included people with cirrhosis due to varied aetiologies, with or without other features of decompensation, having ascites with low protein or previous history of spontaneous bacterial peritonitis. The follow-up in the trials ranged from 1 to 12 months. Many of the trials were at high risk of bias, and the overall certainty of evidence was low or very low. Overall, approximately 10% of trial participants developed spontaneous bacterial peritonitis and 15% of trial participants died. There was no evidence of differences between any of the antibiotics and no intervention in terms of mortality (very low certainty) or number of serious adverse events (very low certainty). However, because of the wide CrIs, clinically important differences in these outcomes cannot be ruled out. None of the trials reported health-related quality of life or the proportion of people with serious adverse events. There was no evidence of differences between any of the antibiotics and no intervention in terms of proportion of people with 'any adverse events' (very low certainty), liver transplantation (very low certainty), or the proportion of people who developed spontaneous bacterial peritonitis (very low certainty). The number of 'any' adverse events per participant was fewer with norfloxacin (rate ratio 0.74, 95% CrI 0.59 to 0.94; 4 trials, 546 participants; low certainty) and sulfamethoxazole plus trimethoprim (rate ratio 0.19, 95% CrI 0.02 to 0.81; 1 trial, 60 participants; low certainty) versus no active intervention. There was no evidence of differences between the other antibiotics and no intervention in the number of 'any' adverse events per participant (very low certainty). There were fewer other decompensation events with rifaximin versus no active intervention (rate ratio 0.61, 65% CrI 0.46 to 0.80; 3 trials, 575 participants; low certainty) and norfloxacin plus neomycin (rate ratio 0.06, 95% CrI 0.00 to 0.33; 1 trial, 22 participants; low certainty). There was no evidence of differences between the other antibiotics and no intervention in the number of decompensations events per participant (very low certainty). None of the trials reported health-related quality of life or development of symptomatic spontaneous bacterial peritonitis. One would expect some correlation between the above outcomes, with interventions demonstrating effectiveness across several outcomes. This was not the case. The possible reasons for this include sparse data and selective reporting bias, which makes the results unreliable. Therefore, one cannot draw any conclusions from these inconsistent differences based on sparse data. There was no evidence of any differences in the subgroup analyses (performed when possible) based on whether the prophylaxis was primary or secondary.
Funding: the source of funding for five trials were organisations who would benefit from the results of the study; six trials received no additional funding or were funded by neutral organisations; and the source of funding for the remaining 18 trials was unclear.
Authors' conclusions: Based on very low-certainty evidence, there is considerable uncertainty about whether antibiotic prophylaxis is beneficial, and if beneficial, which antibiotic prophylaxis is most beneficial in people with cirrhosis and ascites with low protein or history of spontaneous bacterial peritonitis. Future randomised clinical trials should be adequately powered, employ blinding, avoid postrandomisation dropouts (or perform intention-to-treat analysis), and use clinically important outcomes such as mortality, health-related quality of life, and decompensation events.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
KO: none. DR: none. SCF: none. PW: none. AJS: none. NJC: none. CSP: none. EJM: none. NH: none. MC: none. DT: none. BRD: none. ET: none. KSG: none.
Figures




Update of
- doi: 10.1002/14651858.CD013125
References
References to studies included in this review
Abdel Motelleb 2016 {published data only}
-
- Abdel Motelleb MA, Al Azab GE, Alsabaawy MA, Abdel Rasheed MA, Elemam SA, Khodeer SA, et al. Combined norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis. Hepatology International 2016;10(1 Suppl):s390. - PubMed
Ali 2014 {published data only}
-
- Ali B, Zaidi YA, Alam A, Anjum HS. Efficacy of rifaximin in prevention of recurrence of hepatic encephalopathy in patients with cirrhosis of liver. Journal of the College of Physicians and Surgeons Pakistan 2014;24(4):269‐73. - PubMed
Alvarez 2005 {published data only}
-
- Alvarez RF, Mattos AA, Correa EB, Cotrim HP, Nascimento TV. Trimethoprim‐sulfamethoxazole versus norfloxacin in the prophylaxis of spontaneous bacterial peritonitis in cirrhosis. Arquivos de Gastroenterologia 2005;42(4):256‐62. - PubMed
Assem 2016 {published data only}
-
- Assem M, Elsabaawy M, Abdelrashed M, Elemam S, Khodeer S, Hamed W, et al. Efficacy and safety of alternating norfloxacin and rifaximin as primary prophylaxis for spontaneous bacterial peritonitis in cirrhotic ascites: a prospective randomized open‐label comparative multicenter study. Hepatology International 2016;10(2):377‐85. - PubMed
Baik 2015 {published data only}
-
- Baik SK, Lim YL, Cho YZ, Kim MY, Jang YO, Suk KT, et al. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy in the hepatic venous pressure gradient response and propranolol dose reduction‐a pilot study. Journal of Hepatology 2015;62:s188.
-
- Cho YZ, Kim MY, Baik SK, Kwon SO, Kim DJ, Suk KT, et al. Rifaximin and propranolol combination therapy is more effective than propranolol monotherapy in the hepatic venous pressure gradient response and propranolol dose reduction‐a pilot study. Hepatology (Baltimore, Md.) 2014;60:1179a.
Bajaj 2016 {published data only}
-
- Bajaj JS, Flamm SL, Chalasani N, Diaz E, Kayali Z, Kang R, et al. Oral rifaximin soluble solid dispersion immediate‐release 40 mg prevents development of cirrhosis‐related complications: a phase 2, randomized, multicenter, double‐blind, placebo‐controlled trial. Hepatology (Baltimore, Md.) 2016;64(1 Suppl 1):1027a.
-
- Sanyal AJ, Chalasani N, Bajaj JS, Diaz E, Kayali Z, Harmon M, et al. Impact of baseline chronic liver disease characteristics on the efficacy of oral rifaximin soluble solid dispersion tablets for the prevention of further decompensation or all‐cause mortality in patients with cirrhosis and ascites. Hepatology (Baltimore, Md.) 2016;64(1 Suppl 1):47a.
Bass 2010 {published data only}
-
- Bass N, Mullen K, Sigal S, Sanyal A, Poordad F, Merchant K. Rifaximin is effective in maintaining remission in hepatic encephalopathy: results of a large, randomized, placebo controlled trial. Journal of Hepatology 2009;50(Suppl 1):s39.
-
- Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, et al. Rifaximin treatment in hepatic encephalopathy. New England Journal of Medicine 2010;362(12):1071‐81. - PubMed
-
- Brown RS, Bass N, Sanyal AJ, Poordad F, Sheikh MY, Mullen KD. Rifaximin significantly improved critical flicker frequency, and time‐weighted CFF correlated with overt hepatic encephalopathy as assessed by Conn score in a 6 month study. Hepatology (Baltimore, Md.) 2009;50(4 Suppl):449a‐50a.
-
- Butterworth RF, Golden PL, Bortey E, Paterson C, Forbes WP. A critical flicker frequency value of 32 Hz predicts recurrence of overt hepatic encephalopathy in a double‐blind, placebo controlled trial of rifaximin in patients with cirrhosis. Hepatology (Baltimore, Md.) 2012;56:916a‐7a.
-
- Flamm SL, Sanyal AJ, Neff GW, Rolleri RL, Barrett AC, Bortey E, et al. Impact of liver disease status and treatment with rifaximin on complications of cirrhosis in a randomized, placebo‐controlled trial. Hepatology (Baltimore, Md.) 2013;58(4 Suppl):861a‐2a.
Bauer 2002 {published data only}
-
- Bauer TM, Follo A, Navasa M, Vila J, Planas R, Clemente G, et al. Daily norfloxacin is more effective than weekly rufloxacin in prevention of spontaneous bacterial peritonitis recurrence. Digestive Diseases and Sciences 2002;47(6):1356‐61. - PubMed
Elfert 2016 {published data only}
-
- Abd‐Elsalam S, Ali LA, Soliman S, Ibrahim S, Elfert A. Randomized controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. Journal of Hepatology 2016;64(2 Suppl 1):s211. - PubMed
-
- Elfert A, Abo Ali L, Soliman S, Ibrahim S, Abd‐Elsalam S. Randomized‐controlled trial of rifaximin versus norfloxacin for secondary prophylaxis of spontaneous bacterial peritonitis. European Journal of Gastroenterology and Hepatology 2016;28(12):1450‐4. - PubMed
Fernandez 2007 {published data only}
-
- Fernandez J, Navasa M, Planas R, Montoliu S, Monfort D, Soriano G, et al. Primary prophylaxis of spontaneous bacterial peritonitis delays hepatorenal syndrome and improves survival in cirrhosis. Gastroenterology 2007;133(3):818‐24. - PubMed
-
- Navasa M, Fernandez J, Montoliu S, Planas R, Monfort D, Soriano G, et al. Randomized, double‐blind placebo‐controlled trial evaluating norfloxacin the primary prophylaxis of spontaneous bacterial peritonitis in cirrhotics with renal impairment, hyponatremia or severe liver failure. Journal of Hepatology 2006;44:s51.
Ginés 1990 {published data only}
-
- Gines P, Rimola A, Planas R, Vargas V, Forne M, Miranda ML, et al. Norfloxacin prevents spontaneous bacterial peritonitis (SBP) in cirrhosis final results of a multicenter double‐blind placebo controlled study. Journal of Hepatology 1990;11(Suppl 2):s26. - PubMed
-
- Ginés P, Rimola A, Planas R, Vargas V, Marco F, Almela M, et al. Norfloxacin prevents spontaneous bacterial peritonitis recurrence in cirrhosis: Results of a double‐blind, placebo‐controlled trial. Hepatology (Baltimore, Md.) 1990;12(4 pt 1):716‐24. - PubMed
Grangie 1998 {published data only}
-
- Grangie JD, Roulot D, Pelletier G, Pariente EA, Denis J, Ink O, et al. Norfloxacin primary prophylaxis of bacterial infections in cirrhotic patients with ascites: a double‐blind randomized trial. Journal of Hepatology 1998;29(3):430‐6. - PubMed
Ibrahim 2017 {published data only}
-
- Ibrahim ES, Alsebaey A, Zaghla H, Moawad Abdelmageed S, Gameel K, Abdelsameea E. Long‐term rifaximin therapy as a primary prevention of hepatorenal syndrome. European Journal of Gastroenterology and Hepatology 2017;29(11):1247‐50. - PubMed
Kimer 2017 {published data only}
-
- Kimer N, Pedersen JS, Busk TM, Gluud LL, Hobolth L, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis: a randomized, double‐blind, placebo‐controlled trial. Hepatology (Baltimore, Md.) 2017;65(2):592‐603. - PubMed
-
- Kimer N, Pedersen JS, Busk TM, Hobolth L, Gluud LL, Krag A, et al. Rifaximin has no effect on hemodynamics in decompensated cirrhosis – a randomized, double blind, placebo controlled trial. Hepatology (Baltimore, Md.) 2016;64(1 Suppl 1):1041a. - PubMed
-
- Kimer N, Pedersen JS, Moller S, Krag A, Bendtsen F. Randomized trial with rifaximin in liver cirrhosis. Effects on the haemodynamic and inflammatory state. Journal of Hepatology 2015;62:s858.
-
- Kimer N, Pedersen JS, Tavenier J, Christensen JE, Busk TM, Hobolth L, et al. Rifaximin has limited effect on the inflammatory state and bacterial translocation in the patient with stable decompensated cirrhosis. Results from a randomized, placebo controlled trial. Hepatology (Baltimore, Md.) 2016;64(1 Suppl 1):1041a‐2a.
Latif 2016 {published data only}
-
- Latif S, Rashid Siraj M, Khan FS, Sarwar S. Role of oral antibiotics neomycin vs ciprofloxacin for secondary prophylaxis of porto‐systemic encephalopathy in liver cirrhotic patients. Pakistan Journal of Medical and Health Sciences 2016;10(1):32‐6.
Lontos 2014 {published data only}
-
- Lontos S, Gow PJ, Roberts SK, Vaughan RB, Angus PW. Spontaneous bacterial peritonitis (SBP) prophylaxis: a randomised controlled trial comparing trimethoprim‐sulfamethoxazole and norfloxacin. Journal of Hepatology 2008;48(Suppl 2):s119.
-
- Lontos S, Shelton E, Angus PW, Vaughan R, Roberts SK, Gordon A, et al. A randomized controlled study of trimethoprim‐sulfamethoxazole versus norfloxacin for the prevention of infection in cirrhotic patients. Journal of Digestive Diseases 2014;15(5):260‐7. - PubMed
Madrid 2001 {published data only}
-
- Madrid AM, Hurtado C, Venegas M, Cumsille F, Defilippi C. Long‐term treatment with cisapride and antibiotics in liver cirrhosis: effect on small intestinal motility, bacterial overgrowth, and liver function. American Journal of Gastroenterology 2001;96(4):1251‐5. - PubMed
Miglio 1997 {published data only}
-
- Miglio F, Valpiani D, Rossellini SR, Ferrieri A. Rifaximin, a non‐absorbable rifamycin, for the treatment of hepatic encephalopathy. A double‐blind, randomised trial. Current Medical Research and Opinion 1997;13(10):593‐601. - PubMed
Moreau 2015 {published data only}
-
- Moreau R, Elkrief L, Bureau C, Perarnau JM, Thevenot T, Saliba F, et al. Effects of norfloxacin therapy on survival in patients with Child‐Pugh class C cirrhosis: results of a randomized, double‐blind, placebo‐controlled, multicenter trial. Hepatology (Baltimore, Md.) 2015;62(Suppl):282a‐3a.
Mostafa 2014 {published data only}
Mostafa 2015 {published data only}
-
- Mostafa T, Badra G, Abdallah M. The efficacy and the immunomodulatory effect of rifaximin in prophylaxis of spontaneous bacterial peritonitis in cirrhotic Egyptian patients. Turkish Journal of Gastroenterology 2015;26(2):163‐9. - PubMed
Nawaz 2015 {published data only}
-
- Nawaz A, Ghafoor A, Khan A, Ullah H. Rifaximin versus placebo in reducing the risk of clinically overt hepatic encephalopathy recurrence in patients having cirrhosis of liver. Hepatology International 2015;9(1 Suppl 1):s104‐s5.
Praharaj 2017 {published data only}
-
- Praharaj D. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Hepatology International 2018;12(2):S553‐s4.
-
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis (SBP) in cirrhotic patients. Journal of Clinical and Experimental Hepatology 2017;7(Suppl 2):s71.
-
- Praharaj D, Taneja S, Duseja A, Chawla YK, Dhiman RK. Randomized control trial of rifaximin and norfloxacin in primary and secondary prophylaxis of spontaneous bacterial peritonitis in cirrhotic patients. Indian Journal of Gastroenterology 2017;36(1 Suppl 1):a57‐a8.
Rolachon 1995 {published data only}
-
- Rolachon A, Cordier L, Bacq Y, Nousbaum JB, Franza A, Paris JC, et al. Ciprofloxacin and long‐term prevention of spontaneous bacterial peritonitis: results of a prospective controlled trial. Hepatology (Baltimore, Md.) 1995;22(4 pt 1):1171‐4. - PubMed
Singh 1995 {published data only}
-
- Singh N, Gayowski T, Yu VL, Wagener MM. Trimethoprim‐sulfamethoxazole for the prevention of spontaneous bacterial peritonitis in cirrhosis: a randomized trial. Annals of Internal Medicine 1995;122(8):595‐8. - PubMed
Tellez‐Avila 2014 {published data only}
-
- Tellez‐Avila F, Sifuentes‐Osornio J, Barbero‐Becerra V, Franco‐Guzman A, Ruiz‐Cordero R, Alfaro‐Lara R, et al. Primary prophylaxis with ciprofloxacin in cirrhotic patients with ascites: a randomized, double blind study. Annals of Hepatology 2014;13(1):65‐74. - PubMed
Terg 2008 {published data only}
-
- Terg R, Fassio E, Guevara M, Cartier M, Longo C, Lucero R, et al. Ciprofloxacin in primary prophylaxis of spontaneous bacterial peritonitis: a randomized, placebo‐controlled study. Journal of Hepatology 2008;48(5):774‐9. - PubMed
Trespi 1999 {published data only}
-
- Trespi E, Colla C, Belloni G, Polino MG, Magnani L, Panizza P, et al. Efficacy of rifaximin as a prophylactic agent for the prevention of spontaneous bacterial peritonitis in alcoholic cirrhosis. Italian Journal of Gastroenterology and Hepatology 1999;31:534.
Yim 2018 {published data only}
-
- Yim HJ, Suh SJ, Jung YK, Yim SY, Seo YS, Lee YR, et al. Daily norfloxacin vs. weekly ciprofloxacin to prevent spontaneous bacterial peritonitis: a randomized controlled trial. American Journal of Gastroenterology 2018;113(8):1167‐76. - PubMed
-
- Yim HJ, Suh SJ, Jung YK, Yim SY, Seo YS, Park SY, et al. Comparison of daily norfloxacin versus weekly ciprofloxacin for the prevention of spontaneous bacterial peritonitis in cirrhotic patients: a randomized controlled trial. Journal of Hepatology 2016;64(2 Suppl 1):s144.
References to studies excluded from this review
Anonymous 1971 {published data only}
-
- Anonymous. Clinical study of a new nonfungal antibiotic: the trimethoprim‐sulfamethoxazole combination in gastroenterology. Revue Medico‐Chirurgicale des Maladies du Foie 1971;46(6):265‐70. - PubMed
Anonymous 2006 {published data only}
-
- Anonymous. Significance of gastrointestinal antibiotics in gastrointestinal diseases: normix, the non‐absorbable antibiotic. Orvosi Hetilap 2006;147(42):2055‐6. - PubMed
Assy 2005 {published data only}
Bajaj 2012 {published data only}
-
- Bajaj J, Bortey E, Paterson C, Forbes WP. Long‐term use of rifaximin is associated with reduced hospitalizations, prolonged remission: a placebo crossover analysis. Hepatology (Baltimore, Md.) 2012;56(Suppl):925a‐6a.
Bendtsen 2005 {published data only}
-
- Bendtsen F. Increased survival following antibiotic prophylaxis in patients with cirrhosis and upper gastrointestinal hemorrhage. Ugeskrift for Laeger 2005;167(7):733. - PubMed
Boccardi 1974 {published data only}
-
- Boccardi V, Attina D, Girelli G. Influence of orally administered antibiotics on anti‐T agglutinin of normal subjects and of cirrhotic patients. Vox Sanguinis 1974;27(3):268‐72. - PubMed
Bode 1997 {published data only}
-
- Bode C, Schafer C, Fukui H, Bode JC. Effect of treatment with paromomycin on endotoxemia in patients with alcoholic liver disease – a double‐blind, placebo‐controlled trial. Alcoholism, Clinical and Experimental Research 1997;21(8):1367‐73. - PubMed
Gerbes 1997 {published data only}
-
- Gerbes AL. Antibiotic prophylaxis after gastrointestinal hemorrhage in liver cirrhosis – for which patients, with what antibiotics?. Zeitschrift für Gastroenterologie 1997;35(8):659‐60. - PubMed
Gines 1998 {published data only}
-
- Gines P, Navasa M. Antibiotic prophylaxis for spontaneous bacterial peritonitis: how and whom?. Journal of Hepatology 1998;29(3):490‐4. - PubMed
Gupta 2013 {published data only}
-
- Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic vsl#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver International 2013;33(8):1148‐57. - PubMed
Henrion 1992 {published data only}
-
- Henrion J, Schapira M, Derue G, Heller FR. Prevention of bacterial infection using selective intestinal decontamination in patients with cirrhosis admitted to intensive care. Controlled study in 120 patients. Acta Gastro‐enterologica Belgica 1992;55(4):333‐40. - PubMed
Jalan 2010 {published data only}
-
- Jalan R. Rifaximin in hepatic encephalopathy: more than just a non‐absorbable antibiotic?. Journal of Hepatology 2010;53(3):580‐2. - PubMed
Kemp 2009 {published data only}
-
- Kemp W, Colman J, Thompson K, Madan A, Vincent M, Chin‐Dusting J, et al. Norfloxacin treatment for clinically significant portal hypertension: results of a randomised double‐blind placebo‐controlled crossover trial. Liver International 2009;29(3):427‐33. - PubMed
Kumar 2014 {published data only}
-
- Kumar N, Kedarisetty CK, Kumar S, Jindal A, Bhadoria AS, Sarin SK. Pentoxifylline therapy for hepatopulmonary syndrome: longer duration is superior to combination with rifaximin a RCT. Hepatology (Baltimore, Md.) 2014;60:379a.
Lata 2005 {published data only}
-
- Lata J, Jurankova J, Husova L, Senkyrik M, Dite P, Dastych M, et al. Factors participating in development of bleeding varices in portal hypertension. Part I: bacterial infection and comparison of intravenous and peroral antibiotics effects – a randomised study. Vnitrni Lekarstvi 2004;50(11):830‐5. - PubMed
-
- Lata J, Jurankova J, Husova L, Senkyrik M, Dite P, Dastych M, et al. Variceal bleeding in portal hypertension: bacterial infection and comparison of efficacy of intravenous and per‐oral application of antibiotics – a randomized trial. European Journal of Gastroenterology and Hepatology 2005;17(10):1105‐10. - PubMed
Novella 1997 {published data only}
-
- Novella M, Sola R, Soriano G, Andreu M, Gana J, Ortiz J, et al. Continuous versus inpatient prophylaxis of the first episode of spontaneous bacterial peritonitis with norfloxacin. Hepatology (Baltimore, Md.) 1997;25(3):532‐6. - PubMed
Pateron 1992 {published data only}
-
- Pateron D, Blaise M, Trinchet JC, Levacher S, Collignon A, Beaugrand M. Systemic antibiotic therapy (SAB) using ofloxacin is an effective prevention of bacterial infections in cirrhotic patients with variceal hemorrhage. Journal of Hepatology 1992;16(Suppl 1):s41. - PubMed
Rimola 1985 {published data only}
-
- Rimola A, Bory F, Teres J, Perez‐Ayuso RM, Arroyo V, Rodes J. Oral, nonabsorbable antibiotics prevent infection in cirrhotics with gastrointestinal hemorrhage. Hepatology (Baltimore, Md.) 1985;5(3):463‐7. - PubMed
Schubert 1991 {published data only}
-
- Schubert ML, Sanyal AJ, Wong ES. Antibiotic prophylaxis for prevention of spontaneous bacterial peritonitis?. Gastroenterology 1991;101(2):550‐2. - PubMed
Siddique 2010 {published data only}
-
- Siddique A, Kowdley K. Gut instinct: rifaximin for the prevention of hepatic encephalopathy. Hepatology (Baltimore, Md.) 2010;52(2):792‐4. - PubMed
Vibert 2008 {published data only}
-
- Vibert J. Efficacy of norfloxacin in the primary prophylaxis of spontaneous bacterial peritonitis on the survival and occurrence of hepatorenal syndrome. Hepato‐Gastro 2008;15(1):74‐5.
References to ongoing studies
Casper 2015 {published data only}
-
- Casper M, Appenrodt B, Grunhage F, Lammert F. The INCA trial (impact of nod2 genotype‐guided antibiotic prevention on survival in patients with liver cirrhosis and ascites): precision medicine for patients with liver cirrhosis and ascites. Zeitschrift für Gastroenterologie 2015;53(11):1350‐2. - PubMed
-
- Casper M, Mengel M, Fuhrmann C, Herrmann E, Reichert M, Appenrodt B, et al. Design of the INCA trial (impact of nod2 genotype‐guided antibiotic prevention on survival in patients with liver cirrhosis and ascites): prospective, double‐blind, randomised clinical trial evaluating the impact of genotype‐stratified care. Journal of Hepatology 2015;62:s847‐s8.
Additional references
Adam 2012
-
- Adam R, Karam V, Delvart V, O'Grady J, Mirza D, Klempnauer J, et al. Evolution of indications and results of liver transplantation in Europe. A report from the European Liver Transplant Registry (ELTR). Journal of Hepatology 2012;57(3):675‐88. - PubMed
Aldred 2014
Bernardi 2010
-
- Bernardi M. Spontaneous bacterial peritonitis: from pathophysiology to prevention. Internal and Emergency Medicine 2010;5(Suppl 1):S37‐44. - PubMed
Best 2018
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. - PubMed
Chaimani 2013
Chan 2013
Chavez‐Tapia 2010
Cohen 2009
-
- Cohen MJ, Sahar T, Benenson S, Elinav E, Brezis M, Soares‐Weiser K. Antibiotic prophylaxis for spontaneous bacterial peritonitis in cirrhotic patients with ascites, without gastro‐intestinal bleeding. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004791.pub2] - DOI - PMC - PubMed
D'Amico 2006
-
- D'Amico G, Garcia‐Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. Journal of Hepatology 2006;44(1):217‐31. - PubMed
D'Amico 2014
-
- D'Amico G, Pasta L, Morabito A, D'Amico M, Caltagirone M, Malizia G, et al. Competing risks and prognostic stages of cirrhosis: a 25‐year inception cohort study of 494 patients. Alimentary Pharmacology & Therapeutics 2014;39(10):1180‐93. - PubMed
de Franchis 2015
-
- Franchis R, Baveno VIF. Expanding consensus in portal hypertension: report of the Baveno VI consensus workshop: stratifying risk and individualizing care for portal hypertension. Journal of Hepatology 2015;63(3):743‐52. - PubMed
Devani 2019
-
- Devani K, Charilaou P, Jaiswal P, Patil N, Radadiya D, Patel P, et al. Trends in hospitalization, acute kidney injury, and mortality in patients with spontaneous bacterial peritonitis. Journal of Clinical Gastroenterology 2019; Vol. 53, issue 2:e68‐e74. [DOI: 10.1097/MCG.0000000000000973] - DOI - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. - PubMed
Dias 2012a
-
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU technical support document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD3‐Heterogeneity.final‐repor... (accessed 17 July 2018).
Dias 2012b
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). scharr.dept.shef.ac.uk/nicedsu/wp‐content/uploads/sites/7/2016/03/TSD1‐I... (accessed 17 July 2018).
Dias 2014
-
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU technical support document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). nicedsu.org.uk/wp‐content/uploads/2016/03/TSD4‐Inconsistency.final_.15Ap... (accessed 17 July 2018). - PubMed
Dias 2016
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta‐analysis of randomised controlled trials, August 2011 (last updated September 2016). www.ncbi.nlm.nih.gov/pubmedhealth/PMH0088912/pdf/PubMedHealth_PMH0088912... (accessed 17 July 2018). - PubMed
DuPont 2015
-
- DuPont HL. Therapeutic effects and mechanisms of action of rifaximin in gastrointestinal diseases. Mayo Clinic Proceedings 2015;90(8):1116‐24. - PubMed
EASL 2010
-
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology 2010;53(3):397‐417. - PubMed
EASL 2018
EuroQol 2018
-
- EuroQol. EQ‐5D Instruments | About EQ‐5D, 2018. euroqol.org/eq‐5d‐instruments/ (accessed 17 July 2018).
Fleming 2008
-
- Fleming KM, Aithal GP, Solaymani‐Dodaran M, Card TR, West J. Incidence and prevalence of cirrhosis in the united kingdom, 1992‐2001: a general population‐based study. Journal of Hepatology 2008;49(5):732‐8. - PubMed
Gleckman 1981
-
- Gleckman R, Blagg N, Joubert DW. Trimethoprim: mechanisms of action, antimicrobial activity, bacterial resistance, pharmacokinetics, adverse reactions, and therapeutic indications. Pharmacotherapy 1981;1(1):14‐20. - PubMed
Goel 2017
-
- Goel A, Rahim U, Nguyen LH, Stave C, Nguyen MH. Systematic review with meta‐analysis: rifaximin for the prophylaxis of spontaneous bacterial peritonitis. Alimentary Pharmacology & Therapeutics 2017;46(11‐12):1029‐36. - PubMed
Gurusamy 2019
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011
-
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
Hutton 2015
-
- Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Annals of Internal Medicine 2015;162(11):777‐84. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997.
Jackson 2014
Kamal 2017
-
- Kamal F, Khan MA, Khan Z, Cholankeril G, Hammad TA, Lee WM, et al. Rifaximin for the prevention of spontaneous bacterial peritonitis and hepatorenal syndrome in cirrhosis: a systematic review and meta‐analysis. European Journal of Gastroenterology & Hepatology 2017;29(10):1109‐17. - PubMed
Khan 2009
-
- Khan J, Pikkarainen P, Karvonen AL, Makela T, Peraaho M, Pehkonen E, et al. Ascites: aetiology, mortality and the prevalence of spontaneous bacterial peritonitis. Scandinavian Journal of Gastroenterology 2009;44(8):970‐4. - PubMed
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Lu 2006
-
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59.
McPherson 2016
-
- McPherson S, Lucey MR, Moriarty KJ. Decompensated alcohol related liver disease: acute management. BMJ (Clinical Research Ed.) 2016;352:i124. - PubMed
Merion 2010
-
- Merion RM. Current status and future of liver transplantation. Seminars in Liver Disease 2010;30(4):411‐21. - PubMed
Mills 2012
-
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
Mokdad 2014
NCBI 2018a
-
- National Center for Biotechnology Information. Liver cirrhosis. www.ncbi.nlm.nih.gov/mesh/68008103 (accessed on 17 July 2018).
NCBI 2018b
-
- National Center for Biotechnology Information. Ascites. www.ncbi.nlm.nih.gov/mesh/68001201 (accessed on 17 July 2018).
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
Nousbaum 2007
-
- Nousbaum JB, Cadranel JF, Nahon P, Khac EN, Moreau R, Thevenot T, et al. Diagnostic accuracy of the multistix 8 sg reagent strip in diagnosis of spontaneous bacterial peritonitis. Hepatology (Baltimore, Md.) 2007;45(5):1275‐81. - PubMed
Optum 2018
-
- Optum. Patient‐reported outcomes | What we do | SF Health Surveys | SF‐36v2 Health Survey, 2018. campaign.optum.com/optum‐outcomes/what‐we‐do/health‐surveys/sf‐36v2‐heal... (accessed on 14 April 2018).
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. - PubMed
Ratib 2015
-
- Ratib S, Fleming KM, Crooks CJ, Walker AJ, West J. Causes of death in people with liver cirrhosis in England compared with the general population: a population‐based cohort study. American Journal of Gastroenterology 2015;110(8):1149‐58. - PubMed
Read 1972
-
- Read AE. Clinical physiology of the liver. British Journal of Anaesthesia 1972;44(9):910‐7. - PubMed
Rimola 2000
-
- Rimola A, Garcia‐Tsao G, Navasa M, Piddock LJ, Planas R, Bernard B, et al. Diagnosis, treatment and prophylaxis of spontaneous bacterial peritonitis: a consensus document. International Ascites Club. Journal of Hepatology 2000;32(1):142‐53. - PubMed
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Runyon 2013
-
- Runyon BA, AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology (Baltimore, Md.) 2013;57(4):1651‐3. - PubMed
Saab 2009
-
- Saab S, Hernandez JC, Chi AC, Tong MJ. Oral antibiotic prophylaxis reduces spontaneous bacterial peritonitis occurrence and improves short‐term survival in cirrhosis: a meta‐analysis. American Journal of Gastroenterology 2009;104(4):993‐1001. - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Savović 2018
Scaglione 2015
-
- Scaglione S, Kliethermes S, Cao G, Shoham D, Durazo R, Luke A, et al. The epidemiology of cirrhosis in the united states: a population‐based study. Journal of Clinical Gastroenterology 2015;49(8):690‐6. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schulz 2010
Setiawan 2016
Severini 1993
-
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B, Statistical Methodology 1993;55(2):533‐40.
Sidhu 2017
Stata 15.1 [Computer program]
-
- StataCorp LLC. Stata. Version 15.1. College Station, TX, USA: StataCorp LLC, 2017.
Tandon 2011
Tsochatzis 2014
-
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet 2014;383(9930):1749‐61. - PubMed
Tsochatzis 2017
-
- Tsochatzis EA, Gerbes AL. Diagnosis and treatment of ascites. Journal of Hepatology 2017;67(1):184‐5. - PubMed
Turner 2012
van Valkenhoef 2012
-
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. - PubMed
Williams 2014
-
- Williams R, Aspinall R, Bellis M, Camps‐Walsh G, Cramp M, Dhawan A, et al. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet 2014;384(9958):1953‐97. - PubMed
Wood 2008
Yepes‐Nunez 2019
-
- Yepes‐Nunez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta‐analysis. Journal of Clinical Epidemiology 2019;115:1‐13. - PubMed

