FAMIN Is a Multifunctional Purine Enzyme Enabling the Purine Nucleotide Cycle
- PMID: 31978345
- PMCID: PMC6978800
- DOI: 10.1016/j.cell.2019.12.017
FAMIN Is a Multifunctional Purine Enzyme Enabling the Purine Nucleotide Cycle
Erratum in
-
FAMIN Is a Multifunctional Purine Enzyme Enabling the Purine Nucleotide Cycle.Cell. 2020 Feb 20;180(4):815. doi: 10.1016/j.cell.2020.02.005. Cell. 2020. PMID: 32084343 Free PMC article. No abstract available.
Abstract
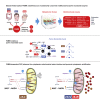
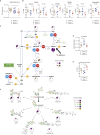
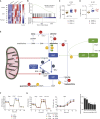
Mutations in FAMIN cause arthritis and inflammatory bowel disease in early childhood, and a common genetic variant increases the risk for Crohn's disease and leprosy. We developed an unbiased liquid chromatography-mass spectrometry screen for enzymatic activity of this orphan protein. We report that FAMIN phosphorolytically cleaves adenosine into adenine and ribose-1-phosphate. Such activity was considered absent from eukaryotic metabolism. FAMIN and its prokaryotic orthologs additionally have adenosine deaminase, purine nucleoside phosphorylase, and S-methyl-5'-thioadenosine phosphorylase activity, hence, combine activities of the namesake enzymes of central purine metabolism. FAMIN enables in macrophages a purine nucleotide cycle (PNC) between adenosine and inosine monophosphate and adenylosuccinate, which consumes aspartate and releases fumarate in a manner involving fatty acid oxidation and ATP-citrate lyase activity. This macrophage PNC synchronizes mitochondrial activity with glycolysis by balancing electron transfer to mitochondria, thereby supporting glycolytic activity and promoting oxidative phosphorylation and mitochondrial H+ and phosphate recycling.
Keywords: C13orf31; Crohn's disease; FAMIN; LACC1; Still's disease; immunometabolism; pH homeostasis; purine metabolism; purine nucleotide cycle; redox homeostasis.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The University of Cambridge has filed patent applications relating to this work. The authors declare no other competing financial interests.
Figures












References
-
- Albers E. Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5′-methylthioadenosine. IUBMB Life. 2009;61:1132–1142. - PubMed
-
- Appleby T.C., Erion M.D., Ealick S.E. The structure of human 5′-deoxy-5′-methylthioadenosine phosphorylase at 1.7 A resolution provides insights into substrate binding and catalysis. Structure. 1999;7:629–641. - PubMed
-
- Aragón J.J., Lowenstein J.M. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur. J. Biochem. 1980;110:371–377. - PubMed
-
- Arinze I.J. Facilitating understanding of the purine nucleotide cycle and the one-carbon pool: Part I: The purine nucleotide cycle. Biochem. Mol. Biol. Educ. 2005;33:165–168. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

