Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration
- PMID: 31978363
- PMCID: PMC7233183
- DOI: 10.1016/j.stem.2019.12.014
Distinct Airway Epithelial Stem Cells Hide among Club Cells but Mobilize to Promote Alveolar Regeneration
Abstract
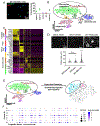
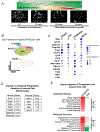
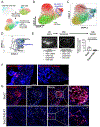
Lung injury activates specialized adult epithelial progenitors to regenerate the epithelium. Depending on the extent of injury, both remaining alveolar type II cells (AEC2s) and distal airway stem/progenitors mobilize to cover denuded alveoli and restore normal barriers. The major source of airway stem/progenitors other than basal-like cells remains uncertain. Here, we define a distinct subpopulation (∼5%) of club-like lineage-negative epithelial progenitors (LNEPs) marked by high H2-K1 expression critical for alveolar repair. Quiescent H2-K1high cells account for virtually all in vitro regenerative activity of airway lineages. After bleomycin injury, H2-K1 cells expand and differentiate in vivo to alveolar lineages. However, injured H2-K1 cells eventually develop impaired self-renewal with features of senescence, limiting complete repair. Normal H2-K1high cells transplanted into injured lungs differentiate into alveolar cells and rescue lung function. These findings indicate that small subpopulations of specialized stem/progenitors are required for effective lung regeneration and are a potential therapeutic adjunct after major lung injury.
Keywords: MHC(high) airway progenitors; alveolar injury and regeneration; bleomycin injury; dedifferentiation; lung epithelial stem cells; oxygenation; single cell transcriptomics; transplantation.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




Comment in
-
A Specialized Few Among Many: Identification of a Novel Lung Epithelial Stem Cell Population.Cell Stem Cell. 2020 Mar 5;26(3):295-296. doi: 10.1016/j.stem.2020.02.010. Cell Stem Cell. 2020. PMID: 32142655 Free PMC article.
References
-
- Brawley C, and Matunis E (2004). Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304, 1331–1334. - PubMed
-
- Brockes JP, and Kumar A (2002). Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol 3, 566–574. - PubMed
-
- Buchweitz JP, Harkema JR, and Kaminski NE (2007). Time-dependent airway epithelial and inflammatory cell responses induced by influenza virus A/PR/8/34 in C57BL/6 mice. Toxicol Pathol 35, 424–435. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

