Immunotherapy for Parkinson's disease
- PMID: 31978602
- PMCID: PMC7933730
- DOI: 10.1016/j.nbd.2020.104760
Immunotherapy for Parkinson's disease
Abstract
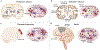
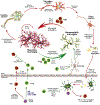
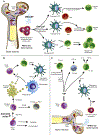
With the increasing prevalence of Parkinson's disease (PD), there is an immediate need to interdict disease signs and symptoms. In recent years this need was met through therapeutic approaches focused on regenerative stem cell replacement and alpha-synuclein clearance. However, neither have shown long-term clinical benefit. A novel therapeutic approach designed to affect disease is focused on transforming the brain's immune microenvironment. As disordered innate and adaptive immune functions are primary components of neurodegenerative disease pathogenesis, this has emerged as a clear opportunity for therapeutic development. Interventions that immunologically restore the brain's homeostatic environment can lead to neuroprotective outcomes. These have recently been demonstrated in both laboratory and early clinical investigations. To these ends, efforts to increase the numbers and function of regulatory T cells over dominant effector cells that exacerbate systemic inflammation and neurodegeneration have emerged as a primary research focus. These therapeutics show broad promise in affecting disease outcomes beyond PD, such as for Alzheimer's disease, stroke and traumatic brain injuries, which share common neurodegenerative disease processes.
Keywords: Alzheimer’s disease; Effector T cells; Granulocyte-macrophage colony stimulating factor; Immune homeostasis; Immune transformation; Ischemic stroke; Neurodegeneration; Neurodegenerative disorders; Neuroinflammation; Neuroprotection; Nigrostriatal degeneration; Parkinson’s disease; Regulatory T cells; Teffs; Traumatic brain injury; Tregs.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None
Figures
References
-
- Bach JF, 2011. Anti-CD3 antibodies for type 1 diabetes: beyond expectations. Lancet. 378, 459–460. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS036126/NS/NINDS NIH HHS/United States
- R01 NS097195/NS/NINDS NIH HHS/United States
- R01 AI145542/AI/NIAID NIH HHS/United States
- P01 DA028555/DA/NIDA NIH HHS/United States
- P30 MH062261/MH/NIMH NIH HHS/United States
- P01 NS031492/NS/NINDS NIH HHS/United States
- P01 DA026146/DA/NIDA NIH HHS/United States
- R56 AI138613/AI/NIAID NIH HHS/United States
- P01 MH064570/MH/NIMH NIH HHS/United States
- R01 AG043540/AG/NIA NIH HHS/United States
- R01 NS070190/NS/NINDS NIH HHS/United States
- R01 NS034239/NS/NINDS NIH HHS/United States
- R01 MH115860/MH/NIMH NIH HHS/United States
- P01 NS043985/NS/NINDS NIH HHS/United States
- R21 NS049264/NS/NINDS NIH HHS/United States
- R01 MH121402/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

