A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients
- PMID: 31978815
- PMCID: PMC7375549
- DOI: 10.1016/j.breast.2020.01.005
A randomized study of olanzapine-containing versus standard antiemetic regimens for the prevention of chemotherapy-induced nausea and vomiting in Chinese breast cancer patients
Abstract
Objectives: Chemotherapy-induced nausea and vomiting (CINV) are distressing symptoms. This randomized study evaluated the antiemetic efficacies of standard antiemetic regimen with/without olanzapine.
Patients and methods: Eligible patients were chemotherapy-naive Chinese breast cancer patients who were planned for (neo)adjuvant doxorubicin/cyclophosphamide. Antiemetic regimen for all studied population included aprepitant, ondansetron and dexamethasone; patients were randomized to Olanzapine (with olanzapine) or Standard arms (without olanzapine). Patients filled in self-reported diaries and completed visual analogue scales for nausea, as well as Functional Living Index-Emesis questionnaires. Blood profiles including fasting glucose and lipids were monitored.
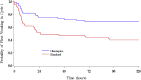
Results: 120 patients were randomized. In Cycle 1 doxorubicin/cyclophosphamide, the Olanzapine arm had significantly higher rates of "Complete Response" than the Standard arm: 65.0% vs 38.3% in the overall period (p = 0.0035), 70.0% vs 51.7% in the acute period (p = 0.0397) and 92.9% vs 74.2% in the delayed period (p = 0.0254). Olanzapine arm also had significantly higher rates of "No significant nausea" and "No nausea" during all 3 time-frames and better QOL. Similar findings were also revealed throughout multiple cycles. Pre-study abnormalities in glucose and lipids occurred in 39.7% and 34.2% of the studied population respectively; there were no differences in these parameters between the two arms at end-of-study assessment.
Conclusion: The addition of olanzapine to standard aprepitant-based antiemetic regimen provides clinically meaningful improvement in controlling CINV. This was associated with a positive impact on QOL and tolerable toxicity profiles among Chinese breast cancer patients receiving doxorubicin/cyclophosphamide chemotherapy. Further studies on metabolic profiles of breast cancer patients are warranted.
Keywords: Aprepitant; Asians; Cyclophosphamide; Dexamethasone; Doxorubicin; Ondansetron; Prospective.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest WY reports consultancy/advisory roles and receipt of personal fees for Novartis, Pfizer, AstraZeneca, Eli Lilly, Roche, Mundipharma and Amgen.
Figures
References
-
- Griffin A.M., Butow P.N., Coates A.S., Childs A.M., Ellis P.M., Dunn S.M., Tattersall M.H. On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol. 1996;7:189–195. - PubMed
-
- Hesketh P.J., Bohlke K., Lyman G., Basch E., Chesney M., Clark-Snow R.A., Danso M.A., Jordan K., Somerfield M.R., Kris M.G., American Society of Clinical Oncology Antiemetics: American society of clinical oncology focused guideline update. J Clin Oncol. 2016;34:381–386. - PubMed
-
- National Comprehensive Cacer Network (NCCN) Clinical practice guidelines in oncology. 28 Feb 2019. http://nccn.org Version 1.2019.
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical