Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI
- PMID: 31978826
- PMCID: PMC6974789
- DOI: 10.1016/j.nicl.2020.102182
Hippocampal plasticity underpins long-term cognitive gains from resistance exercise in MCI
Abstract
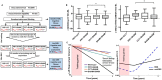
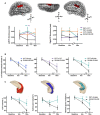
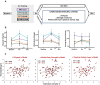
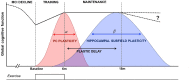
Dementia affects 47 million individuals worldwide, and assuming the status quo is projected to rise to 150 million by 2050. Prevention of age-related cognitive impairment in older persons with lifestyle interventions continues to garner evidence but whether this can combat underlying neurodegeneration is unknown. The Study of Mental Activity and Resistance Training (SMART) trial has previously reported within-training findings; the aim of this study was to investigate the long-term neurostructural and cognitive impact of resistance exercise in Mild Cognitive Impairment (MCI). For the first time we show that hippocampal subareas particularly susceptible to volume loss in Alzheimer's disease (AD) are protected by resistance exercise for up to one year after training. One hundred MCI participants were randomised to one of four training groups: (1) Combined high intensity progressive resistance and computerised cognitive training (PRT+CCT), (2) PRT+Sham CCT, (3) CCT+Sham PRT, (4) Sham physical+sham cognitive training (SHAM+SHAM). Physical, neuropsychological and MRI assessments were carried out at baseline, 6 months (directly after training) and 18 months from baseline (12 months after intervention cessation). Here we report neuro-structural and functional changes over the 18-month trial period and the association with global cognitive and executive function measures. PRT but not CCT or PRT+CCT led to global long-term cognitive improvements above SHAM intervention at 18-month follow-up. Furthermore, hippocampal subfields susceptible to atrophy in AD were protected by PRT revealing an elimination of long-term atrophy in the left subiculum, and attenuation of atrophy in left CA1 and dentate gyrus when compared to SHAM+SHAM (p = 0.023, p = 0.020 and p = 0.027). These neuroprotective effects mediated a significant portion of long-term cognitive benefits. By contrast, within-training posterior cingulate plasticity decayed after training cessation and was unrelated to long term cognitive benefits. Neither general physical activity levels nor fitness change over the 18-month period mediated hippocampal trajectory, demonstrating that enduring hippocampal subfield plasticity is not a simple reflection of post-training changes in fitness or physical activity participation. Notably, resting-state fMRI analysis revealed that both the hippocampus and posterior cingulate participate in a functional network that continued to be upregulated following intervention cessation. Multiple structural mechanisms may contribute to the long-term global cognitive benefit of resistance exercise, developing along different time courses but functionally linked. For the first time we show that 6 months of high intensity resistance exercise is capable of not only promoting better cognition in those with MCI, but also protecting AD-vulnerable hippocampal subfields from degeneration for at least 12 months post-intervention. These findings emphasise the therapeutic potential of resistance exercise; however, future work will need to establish just how long-lived these outcomes are and whether they are sufficient to delay dementia.
Keywords: Hippocampus; Mild cognitive impairment; Plasticity; Randomised controlled trial; Resistance exercise; Subfields.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors have no conflicts of interest to declare.
Figures


References
-
- Alzheimer's Disease International . 2016. World Alzheimer Report 2016. Retrieved from London.
-
- Apostolova L.G., Dutton R.A., Dinov I.D., Hayashi K.M., Toga A.W., Cummings J.L., Thompson P.M. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch. Neurol. 2006;63(5):693–699. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

