Estimating the Clinical and Economic Impact of Switching from the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) to the 10-Valent Pneumococcal Conjugate Vaccine (PCV10) in Italy
- PMID: 31979079
- PMCID: PMC7168640
- DOI: 10.3390/pathogens9020076
Estimating the Clinical and Economic Impact of Switching from the 13-Valent Pneumococcal Conjugate Vaccine (PCV13) to the 10-Valent Pneumococcal Conjugate Vaccine (PCV10) in Italy
Abstract
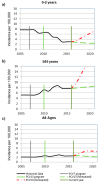
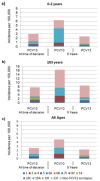
Background: Invasive and non-invasive pneumococcal diseases are significant health and economic burdens, especially in children and the elderly. Italy included the 7-valent (PCV7) and 13-valent pneumococcal conjugate vaccine (PCV13) in the National Immunization Program in 2007 and 2010, respectively, allowing a dramatic reduction in the burden of pneumococcal disease. In the era of budget constraints, decision-makers may consider switching from the higher-valent, more costly PCV13, to the lower-cost PCV10. This study estimated the potential public health and economic impact of changing vaccine programs from PCV13 to PCV10 in Italy. Methods: A decision-analytic forecasting model estimated the impact of PCV programs. Real-world surveillance data were used to forecast serotype distribution and disease incidence among children and the elderly over a specified 5-year time horizon. Costs and outcomes included estimates of cases and deaths avoided, quality-adjusted life years (QALYs) gained, and total costs from a payer perspective, discounted at an assumed rate of 3.0%, and robustness validated through several scenarios and sensitivity analyses. Results: A switch from PCV13 to PCV10 would increase invasive pneumococcal disease (IPD) cases by 59.3% (4317 cases) over a 5-year horizon, primarily due to serotypes 3 and 19A. Pneumonia increased by 8.3% and acute otitis media (AOM) by 96.1%. Maintaining a PCV13 program would prevent a total incremental 531,435 disease cases (1.02M over a 10-year time horizon) and 641 deaths due to invasive pneumococcal disease (IPD), with €23,642 per QALY gained over 5 years versus PCV10. One-way and probabilistic sensitivity analyses showed that a PCV13-based program remained cost-effective in 99.7% of the simulations in Italy as parameters varied within their plausible range; percent vaccinated had the most impact. Conclusions: Maintaining the PCV13 strategy would provide substantial public health and economic benefits in Italy and is cost-effective. Switching from PCV13 to PCV10 would increase the incidence of pneumococcal disease primarily linked to re-emergence of serotypes 3 and 19A.
Keywords: cost-effectiveness analysis; impact; pneumococcal infections; pneumococcal vaccines.
Conflict of interest statement
Filippo Ansaldi, Andrea Orsi and Giancarlo Icardi belong to the organization that received funding from Pfizer in connection with the development of this manuscript. Giancarlo Icardi, Cecilia Trucchi, and Andrea Orsi have been invited as speakers at conferences promoted by Pfizer. Sarah Pugh, Roberto Di Virgilio, and Alessandro Zollo are Pfizer employees. Daniela Amicizia has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organization Pneumococcal vaccines WHO position paper—2012. Wkly. Epidemiol. Rec. 2012;87:129–144. - PubMed
-
- Tsai Y.H., Hsieh M.J., Chang C.J., Wen Y.W., Hu H.C., Chao Y.N., Huang Y.C., Yang C.T., Huang C.C. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old-Taiwan’s PPV vaccination program. Vaccine. 2015;3:2897–2902. doi: 10.1016/j.vaccine.2015.04.068. - DOI - PubMed
LinkOut - more resources
Full Text Sources

