Phenethyl Isothiocyanate Suppresses Stemness in the Chemo- and Radio-Resistant Triple-Negative Breast Cancer Cell Line MDA-MB-231/IR Via Downregulation of Metadherin
- PMID: 31979093
- PMCID: PMC7072670
- DOI: 10.3390/cancers12020268
Phenethyl Isothiocyanate Suppresses Stemness in the Chemo- and Radio-Resistant Triple-Negative Breast Cancer Cell Line MDA-MB-231/IR Via Downregulation of Metadherin
Abstract
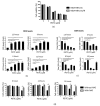
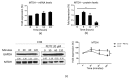
Resistance to chemotherapy and radiation therapy is considered a major therapeutic barrier in breast cancer. Cancer stem cells (CSCs) play a prominent role in chemo and radiotherapy resistance. The established chemo and radio-resistant triple-negative breast cancer (TNBC) cell line MDA-MB-231/IR displays greater CSC characteristics than the parental MDA-MB-231 cells. Escalating evidence demonstrates that metadherin (MTDH) is associated with a number of cancer signaling pathways as well as breast cancer therapy resistance, making it an attractive therapeutic target. Kaplan-Meier plot analysis revealed a correlation between higher levels of MTDH and shorter lifetimes in breast cancer and TNBC patients. Moreover, there was a positive correlation between the MTDH and CD44 expression levels in The Cancer Genome Atlas breast cancer database. We demonstrate that MTDH plays a pivotal role in the regulation of stemness in MDA-MB-231/IR cells. Knockdown of MTDH in MDA-MB-231/IR cells resulted in a reduction in the CSC population, aldehyde dehydrogenase activity, and major CSC markers, including β-catenin, CD44+, and Slug. In addition, MTDH knockdown increased reactive oxygen species (ROS) levels in MDA-MB-231/IR cells. We found that phenethyl isothiocyanate (PEITC), a well-known pro-oxidant phytochemical, suppressed stemness in MDA-MB-231/IR cells through ROS modulation via the downregulation of MTDH. Co-treatment of PEITC and N-Acetylcysteine (a ROS scavenger) caused alterations in PEITC induced cell death and CSC markers. Moreover, PEITC regulated MTDH expression at the post-transcriptional level, which was confirmed using cycloheximide, a protein synthesis inhibitor.
Keywords: cancer stem cells; metadherin; phenethyl isothiocyanate; reactive oxygen species; resistance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures






References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

