Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer
- PMID: 31979186
- PMCID: PMC7073203
- DOI: 10.3390/cancers12020275
Pancreatic Enzyme Replacement Therapy in Pancreatic Cancer
Abstract
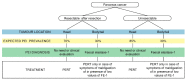
Pancreatic cancer is an aggressive malignancy and the seventh leading cause of global cancer deaths in industrialised countries. More than 80% of patients suffer from significant weight loss at diagnosis and over time tend to develop severe cachexia. A major cause of weight loss is malnutrition. Patients may experience pancreatic exocrine insufficiency (PEI) before diagnosis, during nonsurgical treatment, and/or following surgery. PEI is difficult to diagnose because testing is cumbersome. Consequently, PEI is often detected clinically, especially in non-specialised centres, and treated empirically. In this position paper, we review the current literature on nutritional support and pancreatic enzyme replacement therapy (PERT) in patients with operable and non-operable pancreatic cancer. To increase awareness on the importance of PERT in pancreatic patients, we provide recommendations based on literature evidence, and when data were lacking, based on our own clinical experience.
Keywords: chemotherapy; nutritional support; pancreatic cancer; pancreatic enzyme replacement therapy; pancreatic exocrine insufficiency; pancreatic resection.
Conflict of interest statement
Raffaele Pezzilli is a paid consultant for Mylan Italia S.r.l. Capurso is a paid consultant for Mylan Italia S.r.l. Riccardo Caccialanza received personal fees, speakers’ honoraria, research grants from, or participated on advisory boards for: Akern, Angelini, Baxter Healthcare, B. Braun, Boehringer Ingelheim, Eli Lilly, Fresenius Kabi, Nestlè Health Science, Mylan and Nutricia. Oronzo Brunetti is a paid consultant for Mylan Italia S.r.l. Michele Milella received speakers’ honoraria from and participated on advisory boards for: EUSA Pharma, Pfizer, MSD, AstraZeneca, Merck-Serono and Mylan. Massimo Falconi received speakers’ honoraria and research grants from, or participated on advisory boards for: J&J, Novartis, Ipsen, AAA, Mylan and Celgene. The funder had no role in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- International Agency for Research on Cancer. [(accessed on 1 October 2019)]; Available online: https://gco.iarc.fr/today.
-
- I Numeri del Cancro in Italia 2019. [(accessed on 2 December 2019)]; Available online: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operat....
Publication types
LinkOut - more resources
Full Text Sources

