Desktop Fabrication of Lab-On-Chip Devices on Flexible Substrates: A Brief Review
- PMID: 31979275
- PMCID: PMC7074936
- DOI: 10.3390/mi11020126
Desktop Fabrication of Lab-On-Chip Devices on Flexible Substrates: A Brief Review
Abstract
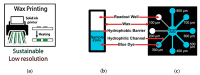
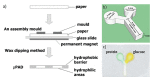
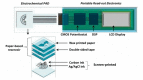
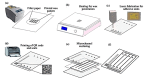
Flexible microfluidic devices are currently in demand because they can be mass-produced in resource-limited settings using simple and inexpensive fabrication tools. Finding new ways to fabricate microfluidic platforms on flexible substrates has been a hot area. Integration of customized detection tools for different lab-on-chip applications has made this area challenging. Significant advancements have occurred in the area over the last decade; therefore, there is a need to review such interesting fabrication tools employed on flexible substrates, such as paper and plastics. In this short review, we review individual fabrication tools and their combinations that have been used to develop such platforms in the past five years. These tools are not only simple and low-cost but also require minimal skills for their operation. Moreover, key examples of plastic-based flexible substrates are also presented, because a diverse range of plastic materials have prevailed recently for a variety of lab-on-chip applications. This review should attract audience of various levels, i.e., from hobbyists to scientists, and from high school students to postdoctoral researchers, to produce their own flexible devices in their own settings.
Keywords: biosensors; desktop fabrication; flexible devices; lab-on-chip; microfluidics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









Similar articles
-
Flexible plastic, paper and textile lab-on-a chip platforms for electrochemical biosensing.Lab Chip. 2018 Jun 26;18(13):1812-1830. doi: 10.1039/c8lc00025e. Lab Chip. 2018. PMID: 29855637 Review.
-
Integrated lab-on-a-chip devices: Fabrication methodologies, transduction system for sensing purposes.J Pharm Biomed Anal. 2023 Jan 20;223:115120. doi: 10.1016/j.jpba.2022.115120. Epub 2022 Oct 22. J Pharm Biomed Anal. 2023. PMID: 36343538 Review.
-
Rapid and inexpensive process to fabricate paper based microfluidic devices using a cut and heat plastic lamination process.Lab Chip. 2022 Sep 13;22(18):3377-3389. doi: 10.1039/d2lc00452f. Lab Chip. 2022. PMID: 35801817
-
Applications of Microfluidics in Liquid Crystal-Based Biosensors.Biosensors (Basel). 2021 Oct 12;11(10):385. doi: 10.3390/bios11100385. Biosensors (Basel). 2021. PMID: 34677341 Free PMC article. Review.
-
Materials for microfluidic chip fabrication.Acc Chem Res. 2013 Nov 19;46(11):2396-406. doi: 10.1021/ar300314s. Epub 2013 Jun 11. Acc Chem Res. 2013. PMID: 24245999
Cited by
-
Microphysiological systems for human aging research.Aging Cell. 2024 Mar;23(3):e14070. doi: 10.1111/acel.14070. Epub 2024 Jan 5. Aging Cell. 2024. PMID: 38180277 Free PMC article. Review.
-
Polymer Microchannel and Micromold Surface Polishing for Rapid, Low-Quantity Polydimethylsiloxane and Thermoplastic Microfluidic Device Fabrication.Polymers (Basel). 2020 Nov 2;12(11):2574. doi: 10.3390/polym12112574. Polymers (Basel). 2020. PMID: 33147807 Free PMC article.
-
Plotter Cut Stencil Masks for the Deposition of Organic and Inorganic Materials and a New Rapid, Cost Effective Technique for Antimicrobial Evaluations.Micromachines (Basel). 2022 Dec 21;14(1):14. doi: 10.3390/mi14010014. Micromachines (Basel). 2022. PMID: 36677074 Free PMC article.
-
DNA interfaces with dimensional materials for biomedical applications.RSC Adv. 2021 Aug 23;11(45):28332-28341. doi: 10.1039/d1ra04917h. eCollection 2021 Aug 16. RSC Adv. 2021. PMID: 35480758 Free PMC article. Review.
-
Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions.Front Bioeng Biotechnol. 2021 Jan 15;8:602659. doi: 10.3389/fbioe.2020.602659. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33520958 Free PMC article. Review.
References
-
- Chen S., Shamsi M.H. Biosensors-on-chip: A topical review. J. Micromech. Microeng. 2017;27:083001. doi: 10.1088/1361-6439/aa7117. - DOI
-
- Printed Sensors Market by Printed Sensor Type: Global Trend and Forecast to 2022. [(accessed on 20 January 2020)]; Available online: https://www.marketsandmarkets.com/Market-Reports/printed-flexible-sensor....
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

