Innovative Three-Step Microwave-Promoted Synthesis of N-Propargyltetrahydroquinoline and 1,2,3-Triazole Derivatives as a Potential Factor Xa (FXa) Inhibitors: Drug Design, Synthesis, and Biological Evaluation
- PMID: 31979319
- PMCID: PMC7037264
- DOI: 10.3390/molecules25030491
Innovative Three-Step Microwave-Promoted Synthesis of N-Propargyltetrahydroquinoline and 1,2,3-Triazole Derivatives as a Potential Factor Xa (FXa) Inhibitors: Drug Design, Synthesis, and Biological Evaluation
Abstract
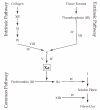
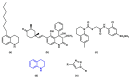
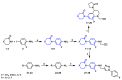
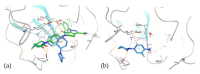
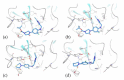
The coagulation cascade is the process of the conversion of soluble fibrinogen to insoluble fibrin that terminates in production of a clot. Factor Xa (FXa) is a serine protease involved in the blood coagulation cascade. Moreover, FXa plays a vital role in the enzymatic sequence which ends with the thrombus production. Thrombosis is a common causal pathology for three widespread cardiovascular syndromes: acute coronary syndrome (ACS), venous thromboembolism (VTE), and strokes. In this research a series of N-propargyltetrahydroquinoline and 1,2,3-triazole derivatives as a potential factor Xa (FXa) inhibitor were designed, synthesized, and evaluated for their FXa inhibitor activity, cytotoxicity activity and coagulation parameters. Rational design for the desired novel molecules was performed through protein-ligand complexes selection and ligand clustering. The microwave-assisted synthetic strategy of selected compounds was carried out by using Ullmann-Goldberg, N-propargylation, Mannich addition, Friedel-Crafts, and 1,3-dipolar cycloaddition type reactions under microwave irradiation. The microwave methodology proved to be an efficient way to obtain all novel compounds in high yields (73-93%). Furthermore, a thermochemical analysis, optimization and reactivity indexes such as electronic chemical potential (µ), chemical hardness (η), and electrophilicity (ω) were performed to understand the relationship between the structure and the energetic behavior of all the series. Then, in vitro analysis showed that compounds 27, 29-31, and 34 exhibited inhibitory activity against FXa and the corresponding half maximal inhibitory concentration (IC50) values were calculated. Next, a cell viability assay in HEK293 and HepG2 cell lines, and coagulation parameters (anti FXa, Prothrombin time (PT), activated Partial Thromboplastin Time (aPTT)) of the most active novel molecules were performed to determine the corresponding cytotoxicity and possible action on clotting pathways. The obtained results suggest that compounds 27 and 29 inhibited FXa targeting through coagulation factors in the intrinsic and extrinsic pathways. However, compound 34 may target coagulation FXa mainly by the extrinsic and common pathway. Interestingly, the most active compounds in relation to the inhibition activity against FXa and coagulation parameters did not show toxicity at the performed coagulation assay concentrations. Finally, docking studies confirmed the preferential binding mode of N-propargyltetrahydroquinoline and 1,2,3-triazole derivatives inside the active site of FXa.
Keywords: 1,2,3-triazole; N-propargyltetrahydroquinoline; cell viability assay; coagulation parameters; factor Xa inhibitors; microwave-assisted synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
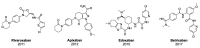



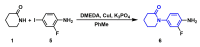
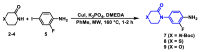
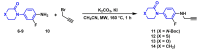
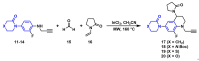
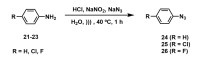



Similar articles
-
Novel Anthranilamide-Based FXa Inhibitors: Drug Design, Synthesis and Biological Evaluation.Molecules. 2016 Apr 14;21(4):491. doi: 10.3390/molecules21040491. Molecules. 2016. PMID: 27089317 Free PMC article.
-
Identification of anthranilamide derivatives as potential factor Xa inhibitors: drug design, synthesis and biological evaluation.Eur J Med Chem. 2015 May 5;95:388-99. doi: 10.1016/j.ejmech.2015.03.052. Epub 2015 Mar 25. Eur J Med Chem. 2015. PMID: 25839438
-
Design, synthesis, and biological activity of novel tetrahydropyrazolopyridone derivatives as FXa inhibitors with potent anticoagulant activity.Bioorg Med Chem. 2017 May 15;25(10):2800-2810. doi: 10.1016/j.bmc.2017.03.055. Epub 2017 Mar 29. Bioorg Med Chem. 2017. PMID: 28389110
-
Advances in the Development of Novel Factor Xa Inhibitors: A Patent Review.Mini Rev Med Chem. 2018;18(16):1332-1353. doi: 10.2174/1389557518666180424120726. Mini Rev Med Chem. 2018. PMID: 29692238 Review.
-
Contemporary medicinal-chemistry strategies for discovery of blood coagulation factor Xa inhibitors.Expert Opin Drug Discov. 2019 Sep;14(9):915-931. doi: 10.1080/17460441.2019.1626821. Epub 2019 Jun 7. Expert Opin Drug Discov. 2019. PMID: 31172842 Review.
Cited by
-
In Vitro Antithrombotic, Hematological Toxicity, and Inhibitor Studies of Protocatechuic, Isovanillic, and p-Hydroxybenzoic Acids from Maclura tricuspidata (Carr.) Bur.Molecules. 2022 May 29;27(11):3496. doi: 10.3390/molecules27113496. Molecules. 2022. PMID: 35684431 Free PMC article.
-
New Hybrid Tetrahydropyrrolo[3,2,1-ij]quinolin-1-ylidene-2-thioxothiazolidin-4-ones as New Inhibitors of Factor Xa and Factor XIa: Design, Synthesis, and In Silico and Experimental Evaluation.Molecules. 2023 May 1;28(9):3851. doi: 10.3390/molecules28093851. Molecules. 2023. PMID: 37175261 Free PMC article.
-
Discovery and development of Factor Xa inhibitors (2015-2022).Front Pharmacol. 2023 Feb 21;14:1105880. doi: 10.3389/fphar.2023.1105880. eCollection 2023. Front Pharmacol. 2023. PMID: 36909153 Free PMC article. Review.
-
Mechanistic and Predictive QSAR Analysis of Diverse Molecules to Capture Salient and Hidden Pharmacophores for Anti-Thrombotic Activity.Int J Mol Sci. 2021 Aug 3;22(15):8352. doi: 10.3390/ijms22158352. Int J Mol Sci. 2021. PMID: 34361118 Free PMC article.
-
Synthesis, Docking, and In Vitro Anticoagulant Activity Assay of Hybrid Derivatives of Pyrrolo[3,2,1-ij]Quinolin-2(1H)-one as New Inhibitors of Factor Xa and Factor XIa.Molecules. 2020 Apr 19;25(8):1889. doi: 10.3390/molecules25081889. Molecules. 2020. PMID: 32325823 Free PMC article.
References
-
- Instituto Nacional de Estadísticas Principales Causas de Muerte en Chile por regiones 1997–2017. [(accessed on 13 December 2019)]; Available online: https://ine.cl/estadisticas/sociales/demografia-y-vitales/nacimientos-ma....
-
- Ministerio de Salud del Gobiernno de Chile Estrategia Nacional de Salud 2011–2020. [(accessed on 13 December 2019)]; Available online: https://www.minsal.cl/portal/url/item/c4034eddbc96ca6de0400101640159b8.pdf.
-
- Ministerio de Salud del Gobiernno de Chile Normas de Seguridad del Paciente y Calidad de Atención Respecto de Prevención Enfermedad Tromboembólica. [(accessed on 13 December 2019)]; Available online: https://www.minsal.cl/portal/url/item/cede67f930f982cce040010164012d43.pdf.
-
- Bombin F. J., Kotlik A.A., Díaz G. A., Vera O. R., Contreras T. J., Vásquez Z. D. Anticoagulant Drugs. Rev. Chil. Cirugía. 2005;57:311–319.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

