Unraveling the complexity of γ-secretase
- PMID: 31980377
- PMCID: PMC7371508
- DOI: 10.1016/j.semcdb.2020.01.005
Unraveling the complexity of γ-secretase
Abstract
γ-Secretase was initially defined as a proteolytic activity that cleaves within the transmembrane of the amyloid precursor protein (APP) to produce the amyloid β-peptide of Alzheimer's disease. The discovery of mutations in APP and the presenilins associated with familial Alzheimer's disease and their effects on APP processing dovetailed with pharmacological studies on γ-secretase, leading to the revelation that presenilins are unprecedented membrane-embedded aspartyl proteases. Other members of what became known as the γ-secretase complex were subsequently identified. In parallel with these advances, connections between presenilins and Notch receptors essential to metazoan development became evident, resulting in the concurrent realization that γ-secretase also carries out intramembrane proteolysis of Notch as part of its signaling mechanism. Substantial progress has been made toward elucidating how γ-secretase carries out complex processing of transmembrane domains, how it goes awry in familial Alzheimer's disease, the scope of its substrates, and the atomic details of its structure. Critical questions remain for future study, toward further unraveling the complexity of this unique membrane-embedded proteolytic machine and its roles in biology and disease.
Keywords: Alzheimer’s disease; Amyloid; Membrane proteins; Notch; Protease.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The author declares no conflicts of interest.
Figures
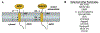

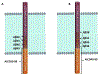

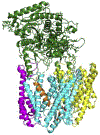
References
-
- Kopan R, and Ilagan MX (2004). γ-Secretase: proteasome of the membrane? Nature reviews. Mol Cell Biol 5, 499–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

