Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study
- PMID: 31980466
- PMCID: PMC7811789
- DOI: 10.1158/1078-0432.CCR-19-3014
Efficacy and Safety of Pembrolizumab in Previously Treated Advanced Neuroendocrine Tumors: Results From the Phase II KEYNOTE-158 Study
Abstract
Purpose: KEYNOTE-158 (ClinicalTrials.gov identifier: NCT02628067) investigated the efficacy and safety of pembrolizumab across multiple cancers. We present results from patients with previously treated advanced well-differentiated neuroendocrine tumors (NET).
Patients and methods: Pembrolizumab 200 mg was administered every 3 weeks for 2 years or until progression, intolerable toxicity, or physician/patient decision. Tumor imaging was performed every 9 weeks for the first year and then every 12 weeks. Endpoints included objective response rate (ORR) per RECIST v1.1 by independent central radiologic review (primary) and duration of response (DOR), progression-free survival (PFS), overall survival (OS), and safety (secondary).
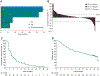
Results: A total of 107 patients with NETs of the lung, appendix, small intestine, colon, rectum, or pancreas were treated. Median age was 59.0 years (range, 29-80), 44.9% had ECOG performance status 1, 40.2% had received ≥3 prior therapies for advanced disease, and 15.9% had PD-L1-positive tumors (combined positive score ≥1). Median follow-up was 24.2 months (range, 0.6-33.4). ORR was 3.7% (95% CI, 1.0-9.3), with zero complete responses and four partial responses (three pancreatic and one rectal) all in patients with PD-L1-negative tumors. Median DOR was not reached, with one of four responses ongoing after ≥21 months follow-up. Median PFS was 4.1 months (95% CI, 3.5-5.4); the 6-month PFS rate was 39.3%. Median OS was 24.2 months (95% CI, 15.8-32.5). Treatment-related adverse events (AE) occurred in 75.7% of patients, 21.5% of whom had grade 3-5 AEs.
Conclusions: Pembrolizumab monotherapy showed limited antitumor activity and manageable safety in patients with previously treated advanced well-differentiated NETs.
©2020 American Association for Cancer Research.
Figures
References
-
- Modlin IM, Oberg K, Chung DC, Jensen RT, de Herder WW, Thakker RV, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9: 61–72. - PubMed
-
- Huguet I, Grossman AB, O’Toole D. Changes in the epidemiology of neuroendocrine tumours. Neuroendocrinology 2017;104:105–11. - PubMed
-
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Neuroendocrinetumors Version 3; 2017. https://oncolife.com.ua/doc/nccn/Neuroendocrine_Tumors.pdf.
-
- Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med 2011;364:501–13. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

