Effects of Pregnancy on the Pharmacokinetics of Metformin
- PMID: 31980499
- PMCID: PMC7076518
- DOI: 10.1124/dmd.119.088435
Effects of Pregnancy on the Pharmacokinetics of Metformin
Abstract
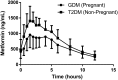
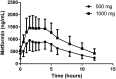
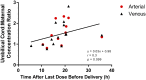
This study's primary objective was to fully characterize the pharmacokinetics of metformin in pregnant women with gestational diabetes mellitus (GDM) versus nonpregnant controls. Steady-state oral metformin pharmacokinetics in pregnant women with GDM receiving either metformin monotherapy (n = 24) or a combination with glyburide (n = 30) as well as in nonpregnant women with type 2 diabetes mellitus (T2DM) (n = 24) were determined utilizing noncompartmental techniques. Maternal and umbilical cord blood samples were collected at delivery from 38 women. With both 500- and 1000-mg doses, metformin bioavailability, volume of distribution beta (V β ), clearance, and renal clearance were significantly increased during pregnancy. In addition, in the women receiving metformin 500 mg, significantly higher metformin apparent oral clearance (CL/F) (27%), weight-adjusted renal secretion clearance (64%), and apparent oral volume of distribution beta (V β /F) (33%) were seen during pregnancy. Creatinine clearance was significantly higher during pregnancy. Increasing metformin dose from 500 to 1000 mg orally twice daily significantly increased V β /F by 28%, weight-adjusted V β /F by 32% and CL/F by 25%, and weight-adjusted CL/F by 28% during pregnancy. Mean metformin umbilical cord arterial-to-venous plasma concentration ratio was 1.0 ± 0.1, venous umbilical cord-to-maternal concentration ratio was 1.4 ± 0.5, and arterial umbilical cord-to-maternal concentration ratio was 1.5 ± 0.5. Systemic exposure after a 500-mg dose of metformin was lower during pregnancy compared with the nonpregnant women with T2DM. However, in patients receiving metformin 1000 mg, changes in estimated bioavailability during pregnancy offset the changes in clearance leading to no significant change in CL/F with the higher dose. SIGNIFICANCE STATEMENT: Gestational diabetes mellitus complicates 5%-13% of pregnancies and is often treated with metformin. Pregnant women undergo physiological changes that alter drug disposition. Preliminary data suggest that pregnancy lowers metformin concentrations, potentially affecting efficacy and safety. This study definitively describes pregnancy's effects on metformin pharmacokinetics and expands the mechanistic understanding of pharmacokinetic changes across the dosage range. Here we report the nonlinearity of metformin pharmacokinetics and the increase in bioavailability, clearance, renal clearance, and volume of distribution during pregnancy.
U.S. Government work not protected by U.S. copyright.
Figures

References
-
- Anderson GD. (2005) Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008. - PubMed
-
- Beckmann R. (1969) [Absorption, distribution in the organism and elimination of metformin]. Diabetologia 5:318–324. - PubMed
-
- Blackhall FH, O’Brien M, Schmid P, Nicolson M, Taylor P, Milenkova T, Kennedy SJ, Thatcher N. (2010) A phase I study of Vandetanib in combination with vinorelbine/cisplatin or gemcitabine/cisplatin as first-line treatment for advanced non-small cell lung cancer. J Thorac Oncol 5:1285–1288. - PubMed
-
- Bryson CL, Ioannou GN, Rulyak SJ, Critchlow C. (2003) Association between gestational diabetes and pregnancy-induced hypertension. Am J Epidemiol 158:1148–1153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

