Pseudomonas aeruginosa lasR mutant fitness in microoxia is supported by an Anr-regulated oxygen-binding hemerythrin
- PMID: 31980538
- PMCID: PMC7022198
- DOI: 10.1073/pnas.1917576117
Pseudomonas aeruginosa lasR mutant fitness in microoxia is supported by an Anr-regulated oxygen-binding hemerythrin
Abstract
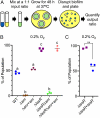
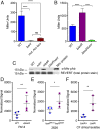
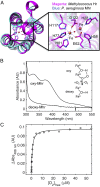
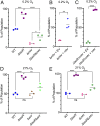
Pseudomonas aeruginosa strains with loss-of-function mutations in the transcription factor LasR are frequently encountered in the clinic and the environment. Among the characteristics common to LasR-defective (LasR-) strains is increased activity of the transcription factor Anr, relative to their LasR+ counterparts, in low-oxygen conditions. One of the Anr-regulated genes found to be highly induced in LasR- strains was PA14_42860 (PA1673), which we named mhr for microoxic hemerythrin. Purified P. aeruginosa Mhr protein contained the predicted di-iron center and bound molecular oxygen with an apparent Kd of ∼1 µM. Both Anr and Mhr were necessary for fitness in lasR+ and lasR mutant strains in colony biofilms grown in microoxic conditions, and the effects were more striking in the lasR mutant. Among genes in the Anr regulon, mhr was most closely coregulated with the Anr-controlled high-affinity cytochrome c oxidase genes. In the absence of high-affinity cytochrome c oxidases, deletion of mhr no longer caused a fitness disadvantage, suggesting that Mhr works in concert with microoxic respiration. We demonstrate that Anr and Mhr contribute to LasR- strain fitness even in biofilms grown in normoxic conditions. Furthermore, metabolomics data indicate that, in a lasR mutant, expression of Anr-regulated mhr leads to differences in metabolism in cells grown on lysogeny broth or artificial sputum medium. We propose that increased Anr activity leads to higher levels of the oxygen-binding protein Mhr, which confers an advantage to lasR mutants in microoxic conditions.
Keywords: Anr; Pseudomonas aeruginosa; hemerythrin; lasR; microoxic growth.
Conflict of interest statement
The authors declare no competing interest.
Figures
Similar articles
-
Links between Anr and Quorum Sensing in Pseudomonas aeruginosa Biofilms.J Bacteriol. 2015 Sep;197(17):2810-20. doi: 10.1128/JB.00182-15. Epub 2015 Jun 15. J Bacteriol. 2015. PMID: 26078448 Free PMC article.
-
Pseudomonas aeruginosa T6SS secretes an oxygen-binding hemerythrin to facilitate competitive growth under microaerobic conditions.Microbiol Res. 2025 Apr;293:128052. doi: 10.1016/j.micres.2025.128052. Epub 2025 Jan 9. Microbiol Res. 2025. PMID: 39813750
-
Conserved C-Terminal Tail Is Responsible for Membrane Localization and Function of Pseudomonas aeruginosa Hemerythrin.Biochemistry. 2024 Jul 16;63(14):1795-1807. doi: 10.1021/acs.biochem.4c00174. Epub 2024 Jul 1. Biochemistry. 2024. PMID: 38951132 Free PMC article.
-
Repair of Iron Center Proteins-A Different Class of Hemerythrin-like Proteins.Molecules. 2022 Jun 23;27(13):4051. doi: 10.3390/molecules27134051. Molecules. 2022. PMID: 35807291 Free PMC article. Review.
-
Bacterial hemerythrin domain-containing oxygen and redox sensors: Versatile roles for oxygen and redox signaling.Front Mol Biosci. 2022 Aug 5;9:967059. doi: 10.3389/fmolb.2022.967059. eCollection 2022. Front Mol Biosci. 2022. PMID: 35992274 Free PMC article. Review.
Cited by
-
The end of the reign of a "master regulator''? A defect in function of the LasR quorum sensing regulator is a common feature of Pseudomonas aeruginosa isolates.mBio. 2024 Mar 13;15(3):e0237623. doi: 10.1128/mbio.02376-23. Epub 2024 Feb 5. mBio. 2024. PMID: 38315035 Free PMC article.
-
Transcriptional Profiling of Pseudomonas aeruginosa Infections.Adv Exp Med Biol. 2022;1386:303-323. doi: 10.1007/978-3-031-08491-1_11. Adv Exp Med Biol. 2022. PMID: 36258077 Review.
-
The histidine kinase NahK regulates denitrification and nitric oxide accumulation through RsmA in Pseudomonas aeruginosa.J Bacteriol. 2025 Jan 31;207(1):e0040824. doi: 10.1128/jb.00408-24. Epub 2024 Dec 11. J Bacteriol. 2025. PMID: 39660891 Free PMC article.
-
Malonate is relevant to the lung environment and induces genome-wide stress responses in Pseudomonas aeruginosa.Res Sq [Preprint]. 2024 Sep 10:rs.3.rs-4870062. doi: 10.21203/rs.3.rs-4870062/v1. Res Sq. 2024. PMID: 39315254 Free PMC article. Preprint.
-
Biofilm Maintenance as an Active Process: Evidence that Biofilms Work Hard to Stay Put.J Bacteriol. 2022 Apr 19;204(4):e0058721. doi: 10.1128/jb.00587-21. Epub 2022 Mar 21. J Bacteriol. 2022. PMID: 35311557 Free PMC article. Review.
References
-
- Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R. L., Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 34, 91–100 (2002). - PubMed
-
- Høiby N., et al. , Pseudomonas aeruginosa and the in vitro and in vivo biofilm mode of growth. Microbes Infect. 3, 23–35 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

