Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype
- PMID: 31980643
- PMCID: PMC6981212
- DOI: 10.1038/s41467-020-14387-w
Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype
Erratum in
-
Publisher Correction: Endothelial progeria induces adipose tissue senescence and impairs insulin sensitivity through senescence associated secretory phenotype.Nat Commun. 2020 Jul 28;11(1):3837. doi: 10.1038/s41467-020-17695-3. Nat Commun. 2020. PMID: 32724181 Free PMC article.
Abstract
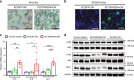
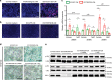
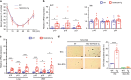
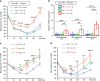
Vascular senescence is thought to play a crucial role in an ageing-associated decline of organ functions; however, whether vascular senescence is causally implicated in age-related disease remains unclear. Here we show that endothelial cell (EC) senescence induces metabolic disorders through the senescence-associated secretory phenotype. Senescence-messaging secretomes from senescent ECs induced a senescence-like state and reduced insulin receptor substrate-1 in adipocytes, which thereby impaired insulin signaling. We generated EC-specific progeroid mice that overexpressed the dominant negative form of telomeric repeat-binding factor 2 under the control of the Tie2 promoter. EC-specific progeria impaired systemic metabolic health in mice in association with adipose tissue dysfunction even while consuming normal chow. Notably, shared circulation with EC-specific progeroid mice by parabiosis sufficiently transmitted the metabolic disorders into wild-type recipient mice. Our data provides direct evidence that EC senescence impairs systemic metabolic health, and thus establishes EC senescence as a bona fide risk for age-related metabolic disease.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

