C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides
- PMID: 31980919
- PMCID: PMC7044259
- DOI: 10.1007/s00253-020-10386-5
C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides
Abstract
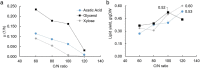
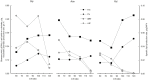
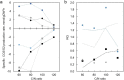
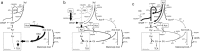
Microbial oils are lipids produced by oleaginous microorganisms, which can be used as a potential feedstock for oleochemical production. The oleaginous yeast Rhodotorula toruloides can co-produce microbial oils and high-value compounds from low-cost substrates, such as xylose and acetic acid (from hemicellulosic hydrolysates) and raw glycerol (a byproduct of biodiesel production). One step towards economic viability is identifying the best conditions for lipid production, primarily the most suitable carbon-to-nitrogen ratio (C/N). Here, we aimed to identify the best conditions and cultivation mode for lipid production by R. toruloides using various low-cost substrates and a range of C/N ratios (60, 80, 100, and 120). Turbidostat mode was used to achieve a steady state at the maximal specific growth rate and to avoid continuously changing environmental conditions (i.e., C/N ratio) that inherently occur in batch mode. Regardless of the carbon source, higher C/N ratios increased lipid yields (up to 60% on xylose at a C/N of 120) but decreased the specific growth rate. Growth on glycerol resulted in the highest specific growth and lipid production (0.085 g lipids/gDW*h) rates at C/Ns between 60 and 100. We went on to study lipid production using glycerol in both batch and fed-batch modes, which resulted in lower specific lipid production rates compared with turbisdostat, however, fed batch is superior in terms of biomass production and lipid titers. By combining the data we obtained in these experiments with a genome-scale metabolic model of R. toruloides, we identified targets for improvements in lipid production that could be carried out either by metabolic engineering or process optimization.
Keywords: Alternative substrates; Biorefineries; Carbon to nitrogen ratio; Flux balance analysis; Genome-scale metabolic model; Microbial oil; Rhodotorula toruloides.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
References
-
- Azambuja SPH, Bonturi N, Miranda EA, Gombert AK (2018) Physiology and lipid accumulation capacity of different Yarrowia lipolytica and Rhodosporidium toruloides strains on glycerol. bioRxiv:1–18. 10.1101/278523
-
- Béligon V, Poughon L, Christophe G, Lebert A, Larroche C, Fontanille P. Validation of a predictive model for fed-batch and continuous lipids production processes from acetic acid using the oleaginous yeast Cryptococcus curvatus. Biochem Eng J. 2016;111:117–128. doi: 10.1016/j.bej.2016.01.016. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

