Cerivastatin for lowering lipids
- PMID: 31981471
- PMCID: PMC6984625
- DOI: 10.1002/14651858.CD012501.pub2
Cerivastatin for lowering lipids
Abstract
Background: Cerivastatin was the most potent statin until it was withdrawn from the market due to a number of fatalities due to rhabdomyolysis, however, the dose-related magnitude of effect of cerivastatin on blood lipids is not known.
Objectives: Primary objective To quantify the effects of various doses of cerivastatin on the surrogate markers: LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides in children and adults with and without cardiovascular disease. The aim of this review is to examine the pharmacology of cerivastatin by characterizing the dose-related effect and variability of the effect of cerivastatin on surrogate markers. Secondary objectives To quantify the effect of various doses of cerivastatin compared to placebo on withdrawals due to adverse effects. To compare the relative potency of cerivastatin with respect to fluvastatin, atorvastatin and rosuvastatin for LDL cholesterol, total cholesterol, HDL cholesterol and triglycerides.
Search methods: The Cochrane Hypertension Information Specialist searched the following databases for RCTs up to March 2019: CENTRAL (2019, Issue 3), Ovid MEDLINE, Ovid Embase, the WHO International Clinical Trials Registry Platform, and ClinicalTrials.gov.We also searched the European Patent Office, FDA.gov, and ProQuest Dissertations & Theses, and contacted authors of relevant papers regarding further published and unpublished work. The searches had no language restrictions.
Selection criteria: RCTs and controlled before-and-after studies evaluating the dose response of different fixed doses of cerivastatin on blood lipids over a duration of three to 12 weeks in participants of any age with and without cardiovascular disease.
Data collection and analysis: Two review authors independently assessed eligibility criteria for trials to be included and extracted data. We entered data from RCTs and controlled before-and-after studies into Review Manager 5 as continuous and generic inverse variance data respectively. We collected information on withdrawals due to adverse effects from the RCTs. We assessed all trials using the 'Risk of bias' tool under the categories of sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting, and other potential biases.
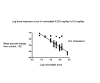
Main results: Fifty trials (19 RCTs and 31 before-and-after studies) evaluated the dose-related efficacy of cerivastatin in 12,877 participants who had their LDL cholesterol measured. The participants were of any age with and without cardiovascular disease and the trials studied cerivastatin effects within a treatment period of three to 12 weeks. Cerivastatin 0.025 mg/day to 0.8 mg/day caused LDL cholesterol decreases of 11.0% to 40.8%, total cholesterol decreases of 8.0% to 28.8% and triglyceride decreases of 9.0% to 21.4%. We judged the certainty of evidence for these effects to be high. Log dose-response data over doses of 2.5 mg to 80 mg revealed strong linear dose-related effects on LDL cholesterol, total cholesterol and triglycerides. When compared to fluvastatin, atorvastatin and rosuvastatin, cerivastatin was about 250-fold more potent than fluvastatin, 20-fold more potent than atorvastatin and 5.5-fold more potent than rosuvastatin at reducing LDL cholesterol; 233-fold more potent than fluvastatin, 18-fold more potent than atorvastatin and six-fold more potent than rosuvastatin at reducing total cholesterol; and 125-fold more potent than fluvastatin, 11-fold more potent than atorvastatin and 13-fold more potent than rosuvastatin at reducing triglycerides. There was no dose-related effect of cerivastatin on HDL cholesterol, but overall cerivastatin increased HDL cholesterol by 5%. There was a high risk of bias for the outcome withdrawals due to adverse effects, but a low risk of bias for the lipid measurements. Withdrawals due to adverse effects were not different between cerivastatin and placebo in 11 of 19 of these short-term trials (risk ratio 1.09, 95% confidence interval 0.68 to 1.74).
Authors' conclusions: The LDL cholesterol, total cholesterol, and triglyceride lowering effect of cerivastatin was linearly dependent on dose. Cerivastatin log dose-response data were linear over the commonly prescribed dose range. Based on an informal comparison with fluvastatin, atorvastatin and rosuvastatin, cerivastatin was about 250-fold more potent than fluvastatin, 20-fold more potent than atorvastatin and 5.5-fold more potent than rosuvastatin in reducing LDL cholesterol, and 233-fold greater potency than fluvastatin, 18-fold greater potency than atorvastatin and six-fold greater potency than rosuvastatin at reducing total cholesterol. This review did not provide a good estimate of the incidence of harms associated with cerivastatin because of the short duration of the trials and the lack of reporting of adverse effects in 42% of the RCTs.
Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Stephen P Adams: none known Nicholas Tiellet: none known Nima Alaeiilkhchi: none known James M Wright: none known
Figures





Update of
References
References to studies included in this review
Amano 1996 {published data only}
-
- Amano K. The clinical effect of novel HMG CoA reductase inhibitor, BAY w 6228, on hyperlipidemia in diabetic patients. Japanese Pharmacology and Therapeutics 1996;24 Suppl 9:253‐62. [EMBASE: 1996316447]
Arakawa 1996 {published data only}
-
- Arakawa K. A comparative and long term trial of a new HMG CoA reductase inhibitor, BAY w 6228 150 mug/day and 300 mug/day in patients with hyperlipidemia. Japanese Pharmacology and Therapeutics. Japan, 1996; Vol. 24 Suppl 9:147‐70. [EMBASE: 1996316441]
Arakawa 1997 {published data only}
-
- Arakawa K. Long‐term trial of a new HMG‐CoA reductase inhibitor, BAY w 6228 (cerivastatin) in patients with hyperlipidemia. Japanese Pharmacology and Therapeutics 1997;25(10):127‐43. [EMBASE: 1998035849]
Balletshofer 2005 {published data only}
-
- Balletshofer BM, Goebbel S, Rittig K, Enderle M, Schmolzer I, Wascher TC, et al. Intense cholesterol lowering therapy with a HMG‐CoA reductase inhibitor does not improve nitric oxide dependent endothelial function in type‐2‐diabetes‐‐a multicenter, randomised, double‐blind, three‐arm placebo‐controlled clinical trial. Experimental and Clinical Endocrinology & Diabetes 2005;113(6):324‐30. [MEDLINE: ] - PubMed
Battula 2000 {published data only}
-
- Battula SB, Fitzsimons O, Moreno S, Owens D, Collins P, Johnson A, et al. Postprandial apolipoprotein B48‐and B100‐containing lipoproteins in type 2 diabetes: do statins have a specific effect on triglyceride metabolism?. Metabolism: Clinical and Experimental 2000;49(8):1049‐54. [MEDLINE: ] - PubMed
Bayer 1992 {published data only}
-
- Bayer. A placebo‐controlled, double‐blind, parallel group 28 day study of the safety and tolerability of BAY w 6228 3000 ug once daily in patients with hypercholesterolemia. Study # D92‐010 (0123)/Report #1139 1998.
-
- Mullican WS. A placebo‐controlled, double‐blind, parallel group 28 day study of the safety and tolerability of BAY w 6228 3000 ug once daily in patients with hypercholesterolemia. Study 0123 (D92‐010) 1992.
Bayer 1994 {published data only}
-
- Bayer. A double‐blind dose ranging study of BAY w 6228 in doses of 50 ug, 100 ug, 200 ug. and 300 ug once daily compared to placebo and to lovastatin 40 mg once daily in patients with hypercholesterolemia. Study # D91‐031 (0124)/ Report # 1304 1998.
-
- Bayer. A double‐blind dose ranging study of BAY w 6228 in doses of 50 ug, 100 ug, 200 ug. and 300 ug once daily compared to placebo and to lovastatin 40 mg once daily in patients with hypercholesterolemia. Study D/X91‐031 1994.
Bayer 1995 {published data only}
-
- Bayer. Efficacy of cerivastatin in FH heterozygotes. Study 0139 1995.
-
- Marais AD. A randomized, double‐blind, parallel group comparison study to compare the efficacy of BAY w 6228 to placebo in the treatment of patients with familial hypercholesterolemia. Study #0139/Report #25062 1999.
Bayer 1997 {published data only}
-
- Bayer. Protocol BAY D97‐008. Study BAY D97‐008 1997.
Bayer 1998 {published data only}
-
- Bayer. NDA:20‐740/S002. Study D96‐008A 1998.
Betteridge 1999 {published data only}
-
- Bayer 1994. A multinational, multicentre, randomised, double‐blind, dose ranging study of BAY w 6228 in doses of 25 ug, 50 ug, 100 ug, 200 ug, once daily compared to placebo and simvastatin 20 mg once daily in patients with hypercholesterolemia. Bayer Study 120 1994. [Study 0120]
-
- Betteridge DJ. International multicentre comparison of cerivastatin with placebo and simvastatin for the treatment of patients with primary hypercholesterolaemia. International Cerivastatin Study Group. International Journal of Clinical Practice 1999;53(4):243‐50. [MEDLINE: ] - PubMed
Chen 2001 {published data only}
-
- Chen Z, Zhang J. Clinical comparison of cerivastatin with simvastatin for aged hyperlipidemia. Chinese Pharmaceutical Journal 2001;36(4):276‐7. [EMBASE: 2001165218]
Davignon 1998 {published data only}
-
- Davignon J, Hanefeld M, Nakaya N, Hunninghake DB, Insull W Jr, Ose L. Clinical efficacy and safety of cerivastatin: summary of pivotal phase IIb/III studies. American Journal of Cardiology 1998;82(4B):32J‐9J. [MEDLINE: ] - PubMed
Dujovne 2000 {published data only}
-
- Bayer. Double blind efficacy and safety study of two doses of Baycol (cerivastatin) and Pravachol (pravastatin) in the treatment of patients with primary hypercholesterolemia (Protocol 001). Cerivastatin Clinical Report Study 001 1998.
-
- Dujovne CA, Knopp R, Kwiterovich P, Hunninghake D, McBride TA, Poland M. Randomized comparison of the efficacy and safety of cerivastatin and pravastatin in 1,030 hypercholesterolemic patients. The Cerivastatin Study Group. Mayo Clinic Proceedings 2000;75(11):1124‐32. [MEDLINE: ] - PubMed
Goto 1996a {published data only}
-
- Goto Y. Clinical evaluation of a new HMG CoA reductase inhibitor, BAY w 6228: a double blind comparative study with pravastatin in patients with hyperlipidemia. Japanese Pharmacology & Therapeutics 1996;24 Suppl 9:263‐96. [EMBASE: 1996316448]
Goto 1996b {published data only}
-
- Goto Y. Clinical efficacy of a new HMG‐CoA reductase inhibitor, BAY w 6228: a dose finding among four groups by the double blind method. Japanese Pharmacology and Therapeutics 1996;24 Suppl 9:87‐115. [EMBASE: 1996316439]
Hanefeld 1999 {published data only}
-
- Bayer. A multinational, multicentre, double‐blind, randomised study of BAY w 6228 in doses of 300 ug and 400 ug once daily compared to placebo in patients with primary hypercholesterolemia. Study #0149 / Report #25068 1995.
-
- Hanefeld M. Effectivity and tolerance of 300 microgram and 400 microgram cerivastatin per day in patients with primary hypercholesterolamia: a European multi‐centre study. Zeitschrift fur Kardiologie 1998;87 Suppl 5:15. [CENTRAL: CN‐00336724 UPDATE]
-
- Hanefeld M, Deslypere JP, Ose L, Durrington PN, Farnier M, Schmage N. Efficacy and safety of 300 micrograms and 400 micrograms cerivastatin once daily in patients with primary hypercholesterolaemia: a multicentre, randomized, double‐blind, placebo‐controlled study. Journal of International Medical Research 1999;27(3):115‐29. [MEDLINE: ] - PubMed
Hunninghake 1998 {published data only}
-
- Balarac N, Betteridge DJ, Klӧr HU. A double‐blind pilot dose‐ranging study of BAY w 6228 in doses of 25 ug, 50 ug, 100 ug, and 200 ug once daily compared to placebo and to simvastatin 20 mg once daily in patients with hyperlipidemia. British Columbia Pharmacare Program 1992; Vol. Study #0110 / Report #23202:275‐82.
-
- Bayer. A double‐blind pilot dose ranging study of BAY w 6228 in doses of 25 ug, 50 ug, 100 ug, and 200 ug once daily compared to placebo and to lovastatin 40 mg once daily in patients with hypercholesterolemia. Study # D91‐012 (0109) / Report #1199 1991.
-
- Bayer. A double‐blind placebo‐controlled dose scheduling study of BAY w 6228 in doses of 100 ug twice daily with breakfast and dinner compared to 200 ug once daily with dinner or at bedtime in patients with hypercholesterolemia. D/X 91‐031 1993.
-
- Bayer. A double‐blind placebo‐controlled dose scheduling study of BAY w 6228 in doses of 100 ug twice daily with breakfast and dinner compared to 200 ug once daily with dinner or at bedtime in patients with hypercholesterolemia. Study #D91‐016 (0111) / Report #1215 1993.
-
- Hunninghake DB. Clinical efficacy of cerivastatin: phase IIa dose‐ranging and dose‐scheduling studies. American Journal of Cardiology 1998;82(4B):26J‐31J. [MEDLINE: ] - PubMed
Hunninghake 2001 {published data only}
-
- Hunninghake D, Insull W, Knopp R, Davidson M, Lohrbauer L, Jones P, et al. Comparison of the efficacy and safety of atorvastatin versus cerivastatin in patients with hypercholesterolemia. Atherosclerosis 1999;144(1):28‐9. [CENTRAL: CN‐00445809] - PubMed
-
- Hunninghake D, Insull W, Knopp R, Davidson M, Lohrbauer L, Jones P, et al. Comparison of the efficacy of atorvastatin versus cerivastatin in primary hypercholesterolemia. American Journal of Cardiology 2001;88(6):635‐9. [MEDLINE: ] - PubMed
Insull 2000 {published data only}
-
- Insull W Jr, Isaacsohn J, Kwiterovich P, Ra P, Brazg R, Dujovne C, et al. Efficacy and safety of cerivastatin 0.8 mg in patients with hypercholesterolaemia: the pivotal placebo‐controlled clinical trial. Cerivastatin Study Group. Journal of International Medical Research 2000;28(2):47‐68. [MEDLINE: ] - PubMed
Isaacsohn 1998 {published data only}
-
- Isaacsohn J, Stein E, Orchard T, Weinstein R, Huh C, Whalen E, et al. Cerivastatin Study Group. Comparative efficacy, safety, and tolerability of cerivastatin a new HMG‐CoA reductase inhibitor, and fluvastatin in subjects with primary hypercholesterolemia. Drugs affecting lipid metabolism: abstract book. Milan, Italy: Milano Fondazione Giovanni Lorenzini, 1998; Vol. Lorenzini Foundation symposium, 273:50.
Kajiyama 1996 {published data only}
-
- Kajiyama G. Effects of BAY w 6228 on biliary lipid metabolism in patients with hyperlipidemia. Japanese Pharmacology and Therapeutics 1996;24(Suppl 9):235‐43. [EMBASE: 1996316445]
Kim 1999 {published data only}
-
- Kim S, Kim DJ, Hahm JR, Kim BJ, Chung JH, Min YK, et al. Cholesterol lowering effect of cerivastatin in Korean patients with primary hypercholesterolemia. Journal of Korean Society of Endocrinology 1999;14(4):729‐38. [CENTRAL: CN‐01045329 UPDATE]
Krone 1999 {published data only}
-
- Krone W, Schmage N, Breuer HW. Comparison of long‐term efficacy and safety of cerivastatin versus pravastatin in primary hypercholesterolaemia. Journal of Drug Assessment 1999;2(Pt 4):327‐40. [CENTRAL: CN‐00281951 UPDATE]
Lankin 2002 {published data only}
-
- Lankin VZ, Tikhaze AK, Konovalova GG, Tutunov VS, Medvedeva NV, Kotkina TI, et al. Intensification of free radical oxidation of low‐density lipoproteins in the plasma of patients with ischemic heart disease receiving beta‐hydroxy‐beta‐methylglutaryl‐coenzyme A reductase inhibitor cerivastatin and inhibition of low‐density lipoprotein peroxidation with antioxidant probucol. Bulletin of Experimental Biology & Medicine 2002;134(1):39‐42. [MEDLINE: ] - PubMed
Ma 2000 {published data only}
-
- Ma P, Hegele R, Yale JF, Schwartz B. CAVEAT: A randomised, double‐blind, parallel group evaluation of cerivastatin 0.4 mg and 0.8 mg compared to atorvastatin 10 mg and 20 mg once daily in patients with combined (type IIb) dyslipidaemia. British Journal of Cardiology 2000;7(12):780‐6. [EMBASE: 2001009668]
-
- Ma, Hegele R, Yale JF, Schwartz B. CAVEAT: a comparison of cerivastatin 0.4 mg and 0.8mg with atorvastatin 10 mg and 20 mg in patients with combined (type IIB) dyslipidaemia. Atherosclerosis Supplements 2001;2(2):48. [MEDLINE: ]
Mabuchi 1998 {published data only}
-
- Mabuchi H, Koizumi J, Kajinami K. Clinical efficacy and safety of cerivastatin in the treatment of heterozygous familial hypercholesterolemia. American Journal of Cardiology 1998;82(4B):52J‐5J. [MEDLINE: ] - PubMed
Matsuo 2005 {published data only}
-
- Matsuo T, Iwade K, Hirata N, Yamashita M, Ikegami H, Tanaka N, et al. Improvement of arterial stiffness by the antioxidant and anti‐inflammatory effects of short‐term statin therapy in patients with hypercholesterolemia. Heart and Vessels 2005;20(1):8‐12. [MEDLINE: ] - PubMed
Matsuzawa 1996 {published data only}
-
- Matsuzawa Y. Clinical evaluation of BAY w 6228, a new HMG CoA reductase inhibitor, in the long term treatment of hyperlipidemia: effect on serum lipids and steroid hormones. Japanese Pharmacology and Therapeutics 1996;24(Suppl 9):117‐45. [EMBASE: 1996316440]
Nakamura 2001 {published data only}
-
- Nakamura T, Ushiyama C, Hirokawa K, Osada S, Shimada N, Koide H. Effect of cerivastatin on urinary albumin excretion and plasma endothelin‐1 concentrations in type 2 diabetes patients with microalbuminuria and dyslipidemia. American Journal of Nephrology 2001;21(6):449‐54. [MEDLINE: ] - PubMed
Nakaya 1996 {published data only}
-
- Nakaya N. Clinical study of Bay w 6228, a new HMG CoA reductase inhibitor, in the long term treatment of hyperlipidemia: comparison between the elderly and younger subjects. Japanese Pharmacology and Therapeutics 1996;24(SUPPL. 9):171‐95. [EMBASE: 1996316442]
Nakaya 1997 {published data only}
-
- Nakaya N. Clinical study of BAY w 6228, a new HMG‐CoA reductase inhibitor, in the long‐term treatment of hyperlipidemia: comparison between the elderly and younger subjects (report II). Japanese Pharmacology and Therapeutics 1997;25(10):145‐69. [EMBASE: 1998035850]
Puccetti 2001 {published data only}
-
- Puccetti L, Bruni F, Bova G, Cercignani M, Palazzuoli A, Console E, et al. Effect of diet and treatment with statins on platelet‐dependent thrombin generation in hypercholesterolemic subjects. Nutrition Metabolism & Cardiovascular Diseases 2001;11(6):378‐87. [MEDLINE: ] - PubMed
Ridker 2001 {published data only}
-
- Ridker PM, Rifai N, Lowenthal SP. Rapid reduction in C‐reactive protein with cerivastatin among 785 patients with primary hypercholesterolemia. Circulation 2001;103(9):1191‐3. [MEDLINE: ] - PubMed
Rubinstein 1999 {published data only}
-
- Rubinstein A, Maritz FJ, Soule SG, Markel A, Chajek‐Shaul T, Maislos M, et al. Efficacy and safety of cerivastatin for type 2 diabetes and hypercholesterolaemia. Hyperlipidaemia in Diabetes Mellitus investigators. Journal of Cardiovascular Risk 1999;6(6):399‐403. [MEDLINE: ] - PubMed
Sakabe 2004 {published data only}
-
- Sakabe K, Fukuda N, Wakayama K, Nada T, Shinohara H, Tamura Y. Time course differences for statin‐induced pleiotropic effects in hypercholesterolemic patients. International Journal of Cardiology 2004;94(1):111‐7. [MEDLINE: ] - PubMed
Sasaki 1998 {published data only}
-
- Sasaki J, Arakawa K, Yamamoto K, Kobori S, Ageta M, Kono S. A comparative long‐term trial of sodium cerivastatin, a new HMG‐CoA reductase inhibitor, in patients presenting with primary hypercholesterolemia [Essai comparatif a long terme de la cerivastatine sodique, un nouvel inhibiteur de l'HMG‐CoA reductase, chez des patients presentant une hypercholesterolemie primaire]. Revue de Medecine Interne 1999;20(Suppl 3):393s‐8s. [MEDLINE: ] - PubMed
-
- Sasaki J, Arakawa K, Yamamoto K, Kobori S, Ageta M, Kono S. A long‐term comparative trial of cerivastatin sodium, a new HMG‐CoA reductase inhibitor, in patients with primary hypercholesterolemia. Clinical Therapeutics 1998;20(3):539‐48. [MEDLINE: ] - PubMed
Saunders 2000 {published data only}
Scharnagl 2004 {published data only}
-
- Scharnagl H, Stojakovic T, Winkler K, Rosinger S, Marz W, Boehm BO. The HMG‐CoA reductase inhibitor cerivastatin lowers advanced glycation end products in patients with type 2 diabetes. Experimental and Clinical Endocrinology & Diabetes 2007;115(6):372‐5. [MEDLINE: ] - PubMed
-
- Scharnagl H, Winkler K, Mantz S, Baumstark MW, Wieland H, Marz W. Inhibition of HMG‐CoA reductase with cerivastatin lowers dense low density lipoproteins in patients with elevated fasting glucose, impaired glucose tolerance and type 2 diabetes mellitus. Experimental and Clinical Endocrinology & Diabetes 2004;112(5):269‐77. [MEDLINE: ] - PubMed
Sebestjen 2002 {published data only}
-
- Sebestjen M, Keber I, Zegura B, Simcic S, Bozic M, Fressart MM, et al. Statin and fibrate treatment of combined hyperlipidemia: the effects on some novel risk factors. Thrombosis and Haemostasis 2004;92(5):1129‐35. [MEDLINE: ] - PubMed
-
- Sebestjen M, Zegura B, Keber I. Both cerivastatin and fenofibrate improve arterial vasoreactivity in patients with combined hyperlipidaemia. Journal of Internal Medicine 2002;251(1):77‐85. [MEDLINE: ] - PubMed
Shinn 2004 {published data only}
-
- Shinn AH. Evaluating the effects of HMG ‐CoA reductase inhibitors on C‐reactive protein, butyrylcholinesterase, and lipids [PhD thesis]. Stockton, California (USA): University of the Pacific, 2004. [ProQuest Document ID: 305146858]
Simons 2002 {published data only}
-
- Simons LA. Additive effect of plant sterol‐ester margarine and cerivastatin in lowering low‐density lipoprotein cholesterol in primary hypercholesterolemia. American Journal of Cardiology 2002;90(7):737‐40. [MEDLINE: ] - PubMed
Solov'eva 1999 {published data only}
-
- Solov'eva E, Rozhkova TA, Tvorogova MG, Kukharchuk VV. Cerivastatin‐a new synthetic 3‐hydroxy‐3‐methylglutaryl (HMG) inhibitor: effect of 0,2 mg dose in patients with primary hyperlipidemias [Tserivastatin‐novyi sinteticheskii ingibitor reduktazy kofermenta A 3‐gidroksi‐3‐metilgliutarila: effekt dozy 0,2 mg u bol'nykh s pervichnymi giperlipidemiiami]. Terapevticheskii Arkhiv 1999;71(8):30‐4. [MEDLINE: ] - PubMed
Stein 1998 {published data only}
-
- Stein E. Cerivastatin in primary hyperlipidemia‐‐a multicenter analysis of efficacy and safety. Atherosclerosis 1998;139(Suppl 1):S15‐22. [MEDLINE: ] - PubMed
-
- Stein E. Cerivastatin in primary hyperlipidemia: a multicenter analysis of efficacy and safety. American Journal of Cardiology 1998;82(4B):40J‐6J. [MEDLINE: ] - PubMed
Stein 1999 {published data only}
-
- Stein E, Isaacsohn J, Stoltz R, Mazzu A, Liu MC, Lane C, et al. Pharmacodynamics, safety, tolerability, and pharmacokinetics of the 0.8‐mg dose of cerivastatin in patients with primary hypercholesterolemia. American Journal of Cardiology 1999;83(10):1433‐6. [MEDLINE: ] - PubMed
Suzuki 2001 {published data only}
-
- Suzuki K. Randomized comparison of the efficacy between cerivastatin and simvastatin in hypercholesterolemia. Therapeutic Research 2001;22(5):1105‐11. [EMBASE: 2002094763]
Tao 2000 {published data only}
-
- Tao P, Wu XS, Yu XJ, Zhu J, Tao SQ. Efficacy and safety of cerivastatin 0.1 mg, 0.2 mg and 0.3 mg in Chinese patients with primary hypercholesterolaemia: a multicentre, randomised, double‐blind, placebo‐controlled study. Journal of Clinical Research 2000;3:29‐40. [EMBASE: 2000231116]
-
- Wang YQ, Jia YH, Tao P. Efficacy and safety of cerivastatin in the treatment of primary hypercholesterolemia: a multicentre, randomized, double‐blind study. Chinese Journal of Cardiology 2000;28(4):259‐63. [CENTRAL: CN‐00361391 UPDATE]
Tazuma 1998 {published data only}
-
- Tazuma S, Yamashita G, Ochi H, Miura H, Kajihara T, Hattori Y, et al. Effects of cerivastatin sodium, a new HMG‐CoA reductase inhibitor, on biliary lipid metabolism in patients with hypercholesterolemia. Clinical Therapeutics 1998;20(3):477‐85. [MEDLINE: ] - PubMed
Wada 1996 {published data only}
-
- Wada H. Effects of BAY w 6228 on coagulation and fibrinolytic systems in patients with hyperlipidemia. Japanese Pharmacology and Therapeutics 1996;24(Suppl 9):243‐52. [EMBASE: 1996316446]
Yu 2002 {published data only}
-
- Yu WC, Chen CH, Tsao HM, Ding YA. A randomized, double‐blind comparison of cerivastatin and lovastatin for treatment of primary hypercholesterolemia. Chung Hua i Hsueh Tsa Chih ‐ Chinese Medical Journal 2002;65(6):260‐7. [MEDLINE: ] - PubMed
References to studies excluded from this review
Anonymous 1998 {published data only}
-
- Anonymous. Cerivastatine‐‐profile of a new lipid‐reducing drug. Internist 1998;39(1 Suppl Cerivastat):1‐8. [MEDLINE: ] - PubMed
Bergstrom 2006 {published data only}
-
- Bergstrom H, Tonstad S. Combined hormone replacement therapy improves lipids but may increase leucocytes. Journal of Internal Medicine 2006;259(5):537‐8. [MEDLINE: ] - PubMed
Deighan 2001 {published data only}
-
- Deighan CJ, Caslake MJ, McConnell M, Boulton‐Jones JM, Packard CJ. Comparative effects of cerivastatin and fenofibrate on the atherogenic lipoprotein phenotype in proteinuric renal disease. Journal of the American Society of Nephrology 2001;12(2):341‐8. [MEDLINE: ] - PubMed
Fegan 2005 {published data only}
-
- Fegan PG, Shore AC, Mawson D, Tooke JE, MacLeod KM. Microvascular endothelial function in subjects with type 2 diabetes and the effect of lipid‐lowering therapy. Diabetic Medicine 2005;22(12):1670‐6. [MEDLINE: ] - PubMed
Fleischmann 2004 {published data only}
-
- Fleischmann EH, John S, Delles C, Schneider MP, Schmidt BM, Schmieder RE. The effect of statins on angiotensin II‐induced hemodynamic changes in young, mildly hypercholesterolemic men. American Journal of Hypertension 2004;17(12 Pt 1):1120‐6. [MEDLINE: ] - PubMed
Fujiwara 2000 {published data only}
-
- Fujiwara A. Effect of cerivastatin on the hypercholesterolemia pretreated with simvastatin. Therapeutic Research 2000;21(2):373‐8. [EMBASE: 2000135385]
Garcia 2002 {published data only}
-
- Garcia I, Errasti P, Lavilla FJ, Ballester B, Manrique J, Rossich E, et al. Effects of cerivastatin in dyslipemia and other cardiovascular risk factors after renal transplantation. Transplantation Proceedings 2002;34(1):401‐2. [MEDLINE: ] - PubMed
Habib 2000 {published data only}
-
- Habib G, Paillard F, Charpentier G, Angellier JF, Roux T, Portal JJ, et al. A multicenter, open‐label, randomized study comparing the efficacy of atorvastatin versus usual care in reducing refractory hypercholesterolemia in high‐risk patients to target levels. Current Therapeutic Research, Clinical and Experimental 2000;61(4):175‐90. [EMBASE: 2000177377]
ISRCTN22144829 {published data only}
-
- ISRCTN22144829. Lipids in Diabetes Study. doi.org/10.1186/ISRCTN22144829 2002.
Koizumi 1996 {published data only}
-
- Koizumi J. The effect of a new HMG CoA reductase inhibitor, BAY w 6228, in combination therapy with colestyramine or probucol for familial hypercholesterolemia (FH). Japanese Pharmacology and Therapeutics 1996;24(Suppl 9):197‐214. [EMBASE: 1996316443]
Lauterbach 2001 {published data only}
-
- Lauterbach KW, Binnen T, Evers T, Harnischmacher U, Ludwig D, Hanrath P, Krone W, Lehmacher W, Leys D, Neuhaus K‐L, Windler E. Primary prevention of stroke: RESPECT. European heart journal, supplement 2000;2(D):D51‐D53. [CENTRAL: CN‐00423870]
Leiter 1999 {published data only}
-
- Leiter LA, Hanna K. Efficacy and safety of cerivastatin in primary hypercholesterolemia: a long term comparative titration study with simvastatin. Canadian Journal of Cardiology 1999;15(5):545‐55. [MEDLINE: ] - PubMed
Leslie 2004 {published data only}
-
- Leslie SJ, Spratt JC, Grieg L, Attina T, Denvir MA, Webb DJ. The effect of cerivastatin therapy on vascular responses to endothelin antagonists in humans. Journal of Cardiovascular Pharmacology 2004;44(Suppl 1):S410‐2. [MEDLINE: ] - PubMed
McPherson 2001 {published data only}
-
- McPherson R, Hanna K, Agro A, Braeken A, Canadian Cerivastatin Study Group. Cerivastatin versus branded pravastatin in the treatment of primary hypercholesterolemia in primary care practice in Canada: a one‐year, open‐label, randomized, comparative study of efficacy, safety, and cost‐effectiveness. Clinical Therapeutics 2001;23(9):1492‐507. [MEDLINE: ] - PubMed
Morisaki 1996 {published data only}
-
- Morisaki N. Long term trial of a new HMG CoA reductase inhibitor, BAY w 6228 in patients with heterozygous familial hypercholesterolemia. Japanese Pharmacology and Therapeutics 1996;24(Suppl 9):215‐34. [EMBASE: 1996316444]
Paniagua 2002 {published data only}
-
- Paniagua JA, Lopez‐Miranda J, Escribano A, Berral FJ, Marin C, Bravo D, et al. Cerivastatin improves insulin sensitivity and insulin secretion in early‐state obese type 2 diabetes. Diabetes 2002;51(8):2596‐603. [MEDLINE: ] - PubMed
Renders 2003 {published data only}
-
- Renders L, Haas CS, Liebelt J, Oberbarnscheidt M, Schocklmann HO, Kunzendorf U. Tacrolimus and cerivastatin pharmacokinetics and adverse effects after single and multiple dosing with cerivastatin in renal transplant recipients. British Journal of Clinical Pharmacology 2003;56(2):214‐9. [MEDLINE: ] - PMC - PubMed
Schmage 2000 {published data only}
-
- Schmage N, Cagatay M, Park SM, Lommerzheim A, Schopen U. Cerivastatin, a novel HMG‐CoA reductase inhibitor: efficacy and tolerability [Cerivastatin, ein neuer HMG‐CoA‐reduktase‐inhibitor: Wirksamkeit und vertraglichkeit]. Herz Kreislauf 2000;32(7‐8):242‐52. [EMBASE: 2000279263]
Tran 2005 {published data only}
-
- Tran D, Lowy A, Howes JB, Howes LG. Effects of cerivastatin on forearm vascular responses, blood pressure responsiveness and ambulatory blood pressure in type 2 diabetic men. Diabetes, Obesity & Metabolism 2005;7(3):273‐81. [MEDLINE: ] - PubMed
Ural 2002 {published data only}
-
- Ural AU, Yilmaz MI, Avcu F, Yalcin A. Treatment with cerivastatin in primary mixed hyperlipidemia induces changes in platelet aggregation and coagulation system components. International Journal of Hematology 2002;76(3):279‐83. [MEDLINE: ] - PubMed
Wilmink 2001 {published data only}
-
- Wilmink HW, Twickler MB, Banga JD, Dallinga‐Thie GM, Eeltink H, Erkelens DW, et al. Effect of statin versus fibrate on postprandial endothelial dysfunction: role of remnant‐like particles. Cardiovascular Research 2001;50(3):577‐82. [MEDLINE: ] - PubMed
Additional references
Adams 2014
Adams 2015
Adams 2018
Bandolier 2004
-
- Bandolier. Cholesterol lowering with statins. Bandolier 2004:121‐2.
Boekholdt 2012
-
- Boekholdt SM, Arsenault BJ, Mora S, Pedersen TR, LaRosaJC, Nestel PJ, et al. Association of LDL cholesterol, non‐HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins: a meta‐analysis. Journal of the American Medical Association 2012;307(12):1302–9. [MEDLINE: ] - PubMed
Bruckert 2005
-
- Bruckert E, Hayem G, Dejager S, Yau C, Begaud B. Mild to moderate muscular symptoms with high‐dosage statin therapy in hyperlipidemic patients‐‐the PRIMO study. Cardiovascular Drugs and Therapy / Sponsored by the International Society of Cardiovascular Pharmacotherapy 2005;19(6):403‐14. [MEDLINE: ] - PubMed
CDC 2011
-
- Centers for Disease Control and Prevention (CDC). Million hearts: strategies to reduce the prevalence of leading cardiovascular disease risk factors‐‐United States, 2011. MMWR ‐ Morbidity & Mortality Weekly Report 2011;60(36):1248‐51. [MEDLINE: ] - PubMed
CTT 2005
-
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Cholesterol Treatment Trialists (CTT) Collaborators. Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267‐78. [MEDLINE: ] - PubMed
Draeger 2006
-
- Draeger A, Monastyrskaya K, Mohaupt M, Hoppeler H, Savolainen H, Allemann C, et al. Statin therapy induces ultrastructural damage in skeletal muscle in patients without myalgia. Journal of Pathology 2006;210:94‐102. - PubMed
Edwards 2003
Furberg 2001
Furukawa 2006
-
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59(1):7‐10. [MEDLINE: ] - PubMed
Gaw 2000
-
- Gaw A, Packard CJ, Shepherd J. Statins: the HMG CoA Reductase Inhibitors in Perspective. London, England: Martin Dunitz Ltd, 2000. [1853174688]
GraphPad Prism 4 [Computer program]
-
- GraphPad Software Inc.. GraphPad Prism 4. Version Version 4.0. La Jolia: GraphPad Software Inc., 2003.
Grundy 2004
-
- Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, ClarkLT, Hunninghake DB, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Journal of the American College of Cardiology 2004;44(3):720–32. [MEDLINE: ] - PubMed
Heran 2008
Higgins 2002
-
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [MEDLINE: ] - PubMed
Higgins 2011
-
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Izquiero‐Palomares 2016
-
- Izquierdo‐Palomares JM, Fernandez‐Tabera JM, Plana MN, Añino Alba A, Gómez Álvarez P, Fernandez‐Esteban I, et al. Chronotherapy versus conventional statins therapy for the treatment of hyperlipidaemia. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD009462.pub2] - DOI - PMC - PubMed
Jaskiewicz 2018
Jaskiewicz 2019
-
- Jaskiewicz, Pajak B, Labieniec‐Watala M, Palma C, Orzechowski A. Diverse action of selected statins on skeletal muscle cells‐an attempt to explain the protective effect of geranylgeraniol (GGOH) in statin‐associated myopathy (SAM). Journal of Clinical Medicine 2019;8(5):694. [DOI: 10.3390/jcm8050694; MEDLINE: ] - DOI - PMC - PubMed
Kaufmann 2006
Kellick 1997
-
- Kellick KA, Burns K, McAndrew E, Haberl E, Hook N, Ellis AK. Focus on atorvastatin: an HMG‐CoA reductase inhibitor for lowering both elevated LDL cholesterol and triglycerides in hypercholesterolemic patients. Formulary 1997;32(4):352‐63. [EMBASE: 1997129035]
Kreatsoulas 2010
Law 2003
Liao 2005
McTaggart 2003
-
- McTaggart F. Comparative pharmacology of rosuvastatin. Atherosclerosis. Supplements 2003;4(1):9‐14. [MEDLINE: ] - PubMed
Moghadasian 1999
-
- Moghadasian MH. Clinical pharmacology of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors. Life Sciences 1999;65(13):1329–37. [MEDLINE: ] - PubMed
Mohaupt 2009
Moher 2009
Morikawa 2005
-
- Morikawa S, Murakami T, Yamazaki H, Izumi A, Saito Y, Hamakubo T, et al. Analysis of the global RNA expression profiles of skeletal muscle cells treated with statins. Journal of Atherosclerosis and Thrombosis 2005;12(3):121‐31. [MEDLINE: ] - PubMed
Muck 2000
Musini 2014
Nishimoto 2003
-
- Nishimoto T, Tozawa R, Amano Y, Wada T, Imura Y, Sugiyama Y. Comparing myotoxic effects of squalene synthase inhibitor, T‐91485, and 3‐hydroxy‐3‐methylglutaryl coenzyme A (HMG‐CoA) reductase inhibitors in human myocytes. Biochemical Pharmacology 2003;66(11):2133‐9. [MEDLINE: ] - PubMed
Page 2019
-
- Page MJ, Higgins JPT, Sterne JAC. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019).Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Plosker 2000
Psaty 2004
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roger 2011
Sakamoto 2013
-
- Sakamoto K, Kimura J. Mechanism of statin‐induced rhabdomyolysis. Journal of Pharmacological Sciences 2013;123(4):289‐94. [MEDLINE: ] - PubMed
Schaefer 2004
-
- Schaefer EJ, McNamara JR, Tayler T, Daly JA, Gleason JL, Seman LJ, et al. Comparisons of effects of statins (atorvastatin, fluvastatin, lovastatin, pravastatin, and simvastatin) on fasting and postprandial lipoproteins in patients with coronary heart disease versus control subjects. American Journal of Cardiology 2004;93(1):31‐9. [MEDLINE: ] - PubMed
Schectman 1996
-
- Schectman G, Hiatt J. Dose‐response characteristics of cholesterol‐lowering drug therapies: implications for treatment. Annals of Internal Medicine 1996;125(12):990‐1000. [MEDLINE: ] - PubMed
Schünemann 2019a
-
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Schünemann 2019b
-
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, Akl EA, Guyatt GH. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. Available from www.training.cochrane.org/handbook.
Thompson 2005
-
- Thompson JF, Man M, Johnson KJ, Wood LS, Lira ME, Lloyd DB, et al. An association study of 43 SNPs in 16 candidate genes with atorvastatin response. Pharmacogenomics Journal 2005;5(6):352‐8. [MEDLINE: ] - PubMed
Tsang 2002
-
- Tsang MB, Adams SP, Jauca C, Wright JM. In some systematic reviews placebos may not be necessary: an example from a statin dose‐response study. 10th Cochrane Colloquium. Stavanger, 31 July‐3 August 2002; Vol. Abstracts:Poster 29.
Urso 2005
-
- Urso ML, Clarkson PM, Hittel D, Hoffman EP, Thompson PD. Changes in ubiquitin proteasome pathway gene expression in skeletal muscle with exercise and statins. Ateriosclerosis, Thrombosis, and Vascular Biology 2005;25:2560‐6. - PubMed
Ward 2007
-
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment (Winchester, England) 2007;11(14):1‐160, iii‐iv. [MEDLINE: ] - PubMed
Yamazaki 2006
-
- Yamazaki H, Suzuki M, Aoki T, Morikawa S, Maejima T, Sato F, et al. Influence of 3‐hydroxy‐3‐methylglutaryl coenzyme A reductase inhibitors on ubiquinone levels in rat skeletal muscle and heart: relationship to cytotoxicity and inhibitory activity for cholesterol synthesis in human skeletal muscle cells. Journal of Atherosclerosis and Thrombosis 2006;13(6):295‐307. [MEDLINE: ] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

