An Optimized Full-Length FLT3/CD3 Bispecific Antibody Demonstrates Potent Anti-leukemia Activity and Reversible Hematological Toxicity
- PMID: 31981494
- PMCID: PMC7054815
- DOI: 10.1016/j.ymthe.2019.12.014
An Optimized Full-Length FLT3/CD3 Bispecific Antibody Demonstrates Potent Anti-leukemia Activity and Reversible Hematological Toxicity
Abstract
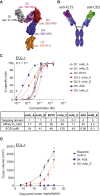
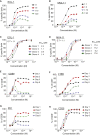
FLT3 (FMS-like tyrosine kinase 3), expressed on the surface of acute myeloid leukemia (AML) blasts, is a promising AML target, given its role in the development and progression of leukemia, and its limited expression in tissues outside the hematopoietic system. Small molecule FLT3 kinase inhibitors have been developed, but despite having clinical efficacy, they are effective only on a subset of patients and associated with high risk of relapse. A durable therapy that can target a wider population of AML patients is needed. Here, we developed an anti-FLT3-CD3 immunoglobulin G (IgG)-based bispecific antibody (7370) with a high affinity for FLT3 and a long half-life, to target FLT3-expressing AML blasts, irrespective of FLT3 mutational status. We demonstrated that 7370 has picomolar potency against AML cell lines in vitro and in vivo. 7370 was also capable of activating T cells from AML patients, redirecting their cytotoxic activity against autologous blasts at low effector-to-target (E:T) ratio. Additionally, under our dosing regimen, 7370 was well tolerated and exhibited potent efficacy in cynomolgus monkeys by inducing complete but reversible depletion of peripheral FLT3+ dendritic cells (DCs) and bone marrow FLT3+ stem cells and progenitors. Overall, our results support further clinical development of 7370 to broadly target AML patients.
Keywords: CD3; FLT3; T cell redirection; acute myeloid leukemia; bispecific; hematopoietic; progenitor.
Copyright © 2020. Published by Elsevier Inc.
Figures




References
-
- Döhner H., Weisdorf D.J., Bloomfield C.D. Acute Myeloid Leukemia. N. Engl. J. Med. 2015;373:1136–1152. - PubMed
-
- Gilliland D.G., Griffin J.D. The roles of FLT3 in hematopoiesis and leukemia. Blood. 2002;100:1532–1542. - PubMed
-
- Gabbianelli M., Pelosi E., Montesoro E., Valtieri M., Luchetti L., Samoggia P., Vitelli L., Barberi T., Testa U., Lyman S. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86:1661–1670. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

