Combining an in silico proarrhythmic risk assay with a tPKPD model to predict QTc interval prolongation in the anesthetized guinea pig assay
- PMID: 31981640
- PMCID: PMC7322544
- DOI: 10.1016/j.taap.2020.114883
Combining an in silico proarrhythmic risk assay with a tPKPD model to predict QTc interval prolongation in the anesthetized guinea pig assay
Abstract
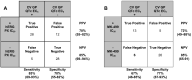
Human-based in silico models are emerging as important tools to study the effects of integrating inward and outward ion channel currents to predict clinical proarrhythmic risk. The aims of this study were 2-fold: 1) Evaluate the capacity of an in silico model to predict QTc interval prolongation in the in vivo anesthetized cardiovascular guinea pig (CVGP) assay for new chemical entities (NCEs) and; 2) Determine if a translational pharmacokinetic/pharmacodynamic (tPKPD) model can improve the predictive capacity. In silico simulations for NCEs were performed using a population of human ventricular action potential (AP) models. PatchXpress® (PX) or high throughput screening (HTS) ion channel data from respectively n = 73 and n = 51 NCEs were used as inputs for the in silico population. These NCEs were also tested in the CVGP (n = 73). An M5 pruned decision tree-based regression tPKPD model was used to evaluate the concentration at which an NCE is liable to prolong the QTc interval in the CVGP. In silico results successfully predicted the QTc interval prolongation outcome observed in the CVGP with an accuracy/specificity of 85%/73% and 75%/77%, when using PX and HTS ion channel data, respectively. Considering the tPKPD predicted concentration resulting in QTc prolongation (EC5%) increased accuracy/specificity to 97%/95% using PX and 88%/97% when using HTS. Our results support that human-based in silico simulations in combination with tPKPD modeling can provide correlative results with a commonly used early in vivo safety assay, suggesting a path toward more rapid NCE assessment with reduced resources, cycle time, and animal use.
Keywords: Anesthetized Cardiovascular Guinea Pig; In Silico modeling; QT corrected interval; Safety pharmacology; Torsade de Pointes; Translational PKPD modeling.
Copyright © 2020 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflicts of interest.
Figures



References
-
- Ahnve S. QT interval prolongation in acute myocardial infarction. Eur. Heart J. 1985;6(Suppl D):85–95. - PubMed
-
- Balasubramanian B., Imredy J.P., Kim D., Penniman J., Lagrutta A., Salata J.J. Optimization of Ca(v)1.2 screening with an automated planar patch clamp platform. J. Pharmacol. Toxicol. Methods. 2009;59:62–72. - PubMed
-
- Beattie K.A., Luscombe C., Williams G., Munoz-Muriedas J., Gavaghan D.J., Cui Y., Mirams G.R. Evaluation of an in silico cardiac safety assay: using ion channel screening data to predict QT interval changes in the rabbit ventricular wedge. J. Pharmacol. Toxicol. Methods. 2013;68:88–96. - PMC - PubMed
-
- Boudoulas H., Sohn Y.H., O’Neill W., Brown R., Weissler A.M. The QT greater than QS2 syndrome: a new mortality risk indicator in coronary artery disease. Am. J. Cardiol. 1982;50:1229–1235. - PubMed
-
- Brennan T., Fink M., Rodriguez B. Multiscale modelling of drug-induced effects on cardiac electrophysiological activity. Eur. J. Pharm. Sci. 2009;36:62–77. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

