High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: Study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial
- PMID: 31981713
- PMCID: PMC7307310
- DOI: 10.1016/j.cct.2020.105938
High-dose rifapentine with or without moxifloxacin for shortening treatment of pulmonary tuberculosis: Study protocol for TBTC study 31/ACTG A5349 phase 3 clinical trial
Abstract
Introduction: Phase 2 clinical trials of tuberculosis treatment have shown that once-daily regimens in which rifampin is replaced by high dose rifapentine have potent antimicrobial activity that may be sufficient to shorten overall treatment duration. Herein we describe the design of an ongoing phase 3 clinical trial testing the hypothesis that once-daily regimens containing high dose rifapentine in combination with other anti-tuberculosis drugs administered for four months can achieve cure rates not worse than the conventional six-month treatment regimen.
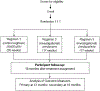
Methods/design: S31/A5349 is a multicenter randomized controlled phase 3 non-inferiority trial that compares two four-month regimens with the standard six-month regimen for treating drug-susceptible pulmonary tuberculosis in HIV-negative and HIV-positive patients. Both of the four-month regimens contain high-dose rifapentine instead of rifampin, with ethambutol replaced by moxifloxacin in one regimen. All drugs are administered seven days per week, and under direct observation at least five days per week. The primary outcome is tuberculosis disease-free survival at twelve months after study treatment assignment. A total of 2500 participants will be randomized; this gives 90% power to show non-inferiority with a 6.6% margin of non-inferiority.
Discussion: This phase 3 trial formally tests the hypothesis that augmentation of rifamycin exposures can shorten tuberculosis treatment to four months. Trial design and standardized implementation optimize the likelihood of obtaining valid results. Results of this trial may have important implications for clinical management of tuberculosis at both individual and programmatic levels.
Trial registration: NCT02410772. Registered 8 April 2015,https://www.clinicaltrials.gov/ct2/show/NCT02410772?term=02410772&rank=1.
Keywords: Moxifloxacin; Multicenter randomized trial; Non-inferiority; Rifapentine; TB; Tuberculosis.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authorship team members have declared any potential conflicts of interest with respect to the research, authorship, and/or publication of this article. Sanofi commercial interests did not influence the study design; the collection, analysis, or interpretation of data; the preparation of this manuscript; or the decision to submit this manuscript for publication. A Sanofi technical expert served on the protocol team.
Figures
References
-
- World Health Organization. Global tuberculosis report 2018. Geneva, Switzerland: World Health Organization; 2018. Report No.: 978-92-4-156564-6.
-
- World Health Organization. Guidelines for treatment of drug-susceptible tuberculosis and patient care, 2017 update. Geneva, Switzerland: World Health Organization; 2017.
-
- Fox W, Ellard GA, Mitchison DA. Studies on the treatment of tuberculosis undertaken by the British Medical Research Council tuberculosis units, 1946-1986, with relevant subsequent publications. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease 1999;3:S231–79. - PubMed
-
- van Ingen J, Aarnoutse RE, Donald PR, et al. Why Do We Use 600 mg of Rifampicin in Tuberculosis Treatment? Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2011;52:e194–9. - PubMed

