Recent advances in tumor associated carbohydrate antigen based chimeric antigen receptor T cells and bispecific antibodies for anti-cancer immunotherapy
- PMID: 31982247
- PMCID: PMC7160013
- DOI: 10.1016/j.smim.2020.101390
Recent advances in tumor associated carbohydrate antigen based chimeric antigen receptor T cells and bispecific antibodies for anti-cancer immunotherapy
Abstract
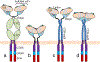
Tumor associated carbohydrate antigens (TACAs) are a class of attractive antigens for the development of anti-cancer immunotherapy. Besides monoclonal antibodies and vaccines, chimeric antigen receptor (CAR) T cells and bispecific antibodies (BsAbs) targeting TACA are exciting directions to harness the power of the immune system to fight cancer. In this review, we focus on two TACAs, i.e., the GD2 ganglioside and the mucin-1 (MUC1) protein. The latest advances in CAR T cells and bispecific antibodies targeting these two antigens are presented. The roles of co-stimulatory molecules, structures of the sequences for antigen binding, methods for CAR and antibody construction, as well as strategies to enhance solid tumor penetration and reduce T cell exhaustion and death are discussed. Furthermore, approaches to reduce "on target, off tumor" side effects are introduced. With further development, CAR T cells and BsAbs targeting GD2 and MUC1 can become powerful agents to effectively treat solid tumor.
Keywords: Bispecific antibody; CAR T cell; Cancer; GD2 ganglioside; Immunotherapy; Mucin-1.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures
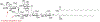
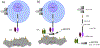

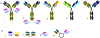
Similar articles
-
Finding the Keys to the CAR: Identifying Novel Target Antigens for T Cell Redirection Immunotherapies.Int J Mol Sci. 2020 Jan 14;21(2):515. doi: 10.3390/ijms21020515. Int J Mol Sci. 2020. PMID: 31947597 Free PMC article. Review.
-
High-Affinity GD2-Specific CAR T Cells Induce Fatal Encephalitis in a Preclinical Neuroblastoma Model.Cancer Immunol Res. 2018 Jan;6(1):36-46. doi: 10.1158/2326-6066.CIR-17-0211. Epub 2017 Nov 27. Cancer Immunol Res. 2018. PMID: 29180536 Free PMC article.
-
Optimization of T Cell Redirecting Strategies: Obtaining Inspirations From Natural Process of T Cell Activation.Front Immunol. 2021 Apr 26;12:664329. doi: 10.3389/fimmu.2021.664329. eCollection 2021. Front Immunol. 2021. PMID: 33981310 Free PMC article. Review.
-
Development of a novel target module redirecting UniCAR T cells to Sialyl Tn-expressing tumor cells.Blood Cancer J. 2018 Aug 22;8(9):81. doi: 10.1038/s41408-018-0113-4. Blood Cancer J. 2018. PMID: 30190468 Free PMC article. No abstract available.
-
Blocking CD38-driven fratricide among T cells enables effective antitumor activity by CD38-specific chimeric antigen receptor T cells.J Genet Genomics. 2019 Aug 20;46(8):367-377. doi: 10.1016/j.jgg.2019.06.007. Epub 2019 Aug 13. J Genet Genomics. 2019. PMID: 31466926
Cited by
-
The cancer glycocode as a family of diagnostic biomarkers, exemplified by tumor-associated gangliosides.Front Oncol. 2023 Oct 26;13:1261090. doi: 10.3389/fonc.2023.1261090. eCollection 2023. Front Oncol. 2023. PMID: 37954075 Free PMC article. Review.
-
Advances in the Immunomodulatory Properties of Glycoantigens in Cancer.Cancers (Basel). 2022 Apr 7;14(8):1854. doi: 10.3390/cancers14081854. Cancers (Basel). 2022. PMID: 35454762 Free PMC article. Review.
-
Tumor-associated antigen-specific cell imaging based on upconversion luminescence and nucleic acid rolling circle amplification.Mikrochim Acta. 2024 Apr 8;191(5):248. doi: 10.1007/s00604-024-06331-2. Mikrochim Acta. 2024. PMID: 38587676
-
Tn Antigen Expression Defines an Immune Cold Subset of Mismatch-Repair Deficient Colorectal Cancer.Int J Mol Sci. 2020 Nov 29;21(23):9081. doi: 10.3390/ijms21239081. Int J Mol Sci. 2020. PMID: 33260328 Free PMC article.
-
Glycoconjugate Nanoparticle-Based Systems in Cancer Immunotherapy: Novel Designs and Recent Updates.Front Immunol. 2022 Mar 30;13:852147. doi: 10.3389/fimmu.2022.852147. eCollection 2022. Front Immunol. 2022. PMID: 35432351 Free PMC article. Review.
References
-
- World Health Organisation, Latest global cancer data, Int. Agency Res. Cancer (2018) 13–15. https://www.iarc.fr/wp-content/uploads/2018/09/pr263_E.pdf (accessed November 3, 2019).
-
- International Agency for Research on Cancer, Cancer Tomorrow, World Heal. Organ (2019)1–2.https://gco.iarc.fr/tomorrow/graphic-line?type=0&population=900&mode=pop... (accessed November 3, 2019).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

