Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis
- PMID: 31982451
- PMCID: PMC7375938
- DOI: 10.1016/j.jaci.2020.01.013
Charcot-Leyden crystal protein/galectin-10 interacts with cationic ribonucleases and is required for eosinophil granulogenesis
Abstract
Background: The human eosinophil Charcot-Leyden crystal (CLC) protein is a member of the Galectin superfamily and is also known as galectin-10 (Gal-10). CLC/Gal-10 forms the distinctive hexagonal bipyramidal crystals that are considered hallmarks of eosinophil participation in allergic responses and related inflammatory reactions; however, the glycan-containing ligands of CLC/Gal-10, its cellular function(s), and its role(s) in allergic diseases are unknown.
Objective: We sought to determine the binding partners of CLC/Gal-10 and elucidate its role in eosinophil biology.
Methods: Intracellular binding partners were determined by ligand blotting with CLC/Gal-10, followed by coimmunoprecipitation and coaffinity purifications. The role of CLC/Gal-10 in eosinophil function was determined by using enzyme activity assays, confocal microscopy, and short hairpin RNA knockout of CLC/Gal-10 expression in human CD34+ cord blood hematopoietic progenitors differentiated to eosinophils.
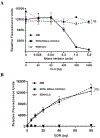
Results: CLC/Gal-10 interacts with both human eosinophil granule cationic ribonucleases (RNases), namely, eosinophil-derived neurotoxin (RNS2) and eosinophil cationic protein (RNS3), and with murine eosinophil-associated RNases. The interaction is independent of glycosylation and is not inhibitory toward endoRNase activity. Activation of eosinophils with INF-γ induces the rapid colocalization of CLC/Gal-10 with eosinophil-derived neurotoxin/RNS2 and CD63. Short hairpin RNA knockdown of CLC/Gal-10 in human cord blood-derived CD34+ progenitor cells impairs eosinophil granulogenesis.
Conclusions: CLC/Gal-10 functions as a carrier for the sequestration and vesicular transport of the potent eosinophil granule cationic RNases during both differentiation and degranulation, enabling their intracellular packaging and extracellular functions in allergic inflammation.
Keywords: Charcot-Leyden; ECP; EDN; Eosinophils; RNase 2; RNase 3; galectins; granulogenesis; ribonucleases.
Copyright © 2020 American Academy of Allergy, Asthma & Immunology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors have no financial or other conflicts of interest to declare.
Figures





References
-
- Charcot JM, Robin C. Observation de Leukocythemia. C R Mem Soc Biol 1853; 5:44.
-
- von Leyden EV. Zur Kenntniss des Bronchial Asthma. [Virchows] Archiv für pathologische Anatomie und Physiologie, und für klinische Medizin 1872; 54:324–44.
-
- Ackerman SJ, Zhou Z-Q, Tenen DG, Clark MA, Tu Y-P, Irvin CG. Human eosinophil lysophospholipase (Charcot-Leyden crystal protein): Molecular cloning, expression, and potential functions in asthma In: Gleich GJ, Kay AB, editors. Eosinophils In Allergy and Inflammation. New York: Marcel Dekker; 1994. p. 21–54.
-
- Ackerman SJ. Characterization and functions of eosinophil granule proteins In: Makino S, Fukuda T, editors. Eosinophils: Biological and Clinical Aspects. Boca Raton: CRC Press; 1993. p. 33–74.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

