Prognostic Value of Circulating Microvesicle Subpopulations in Ischemic Stroke and TIA
- PMID: 31983048
- PMCID: PMC7340656
- DOI: 10.1007/s12975-019-00777-w
Prognostic Value of Circulating Microvesicle Subpopulations in Ischemic Stroke and TIA
Abstract
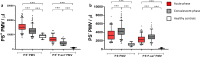
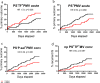
Platelet microvesicles (PMV) have previously been found elevated in acute ischemic stroke (IS) and could be biomarkers for risk of recurrence. PMV surface antigens such as P-selectin and phosphatidylserine (PS) reflect platelet activation and procoagulance. Tissue factor-positive microvesicles (TF+MV) are considered procoagulant, in particular if co-expressing PS. We enumerated MV subpopulations with these surface antigens in a cohort of 211 patients with primarily non-cardioembolic IS or transient ischemic attack (TIA) and investigated their association with long-term outcome. MV concentrations were determined by flow cytometry in the acute and convalescent phase. Primary outcome was a composite of fatal and non-fatal recurrent IS or myocardial infarction. Secondary outcomes were recurrent IS and all-cause mortality. Outcome events were obtained from Swedish registers during a follow-up of 1100 patient years. Concentrations of PS-positive and PS-negative MV populations were elevated in patients compared with healthy controls in both the acute and convalescent phase. PS+TF+PMV displayed pronounced elevations, median fold change 77 in the acute phase (p < 0.0001) but were not associated with outcome, neither were PS+P-selectin+PMV. The only subpopulation positively associated with primary outcome was PS-TF+PMV, with adjusted hazard ratio of 1.86 (1.04-3.31, p = 0.036) by Cox regression. Unexpectedly, several MV subpopulations tended to be associated with reduced risk of poor long-term outcome. Our results suggest that PS+TF+PMV may be a promising marker for cerebral ischemia, and that the in vivo generation of PS-MV after IS/TIA warrants further study. Future MV studies should ideally enumerate PS+ and PS-MV subpopulations separately.
Keywords: Ischemic stroke; Microvesicles; Phosphatidylserine; Platelet; TIA.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- DALYs GBD, Collaborators H Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2018;392(10159):1859–1922. - PMC - PubMed
-
- Grau AJ, Ruf A, Vogt A, Lichy C, Buggle F, Patscheke H, Hacke W. Increased fraction of circulating activated platelets in acute and previous cerebrovascular ischemia. Thromb Haemost. 1998;80(2):298–301. - PubMed
-
- Meiklejohn DJ, Vickers MA, Morrison ER, Dijkhuisen R, Moore I, Urbaniak SJ, Greaves M. In vivo platelet activation in atherothrombotic stroke is not determined by polymorphisms of human platelet glycoprotein IIIa or Ib. Br J Haematol. 2001;112(3):621–631. - PubMed
-
- Garlichs CD, Kozina S, Fateh-Moghadam S, Handschu R, Tomandl B, Stumpf C, Eskafi S, Raaz D, Schmeisser A, Yilmaz A, Ludwig J, Neundörfer B, Daniel WG. Upregulation of CD40-CD40 ligand (CD154) in patients with acute cerebral ischemia. Stroke. 2003;34(6):1412–1418. - PubMed
-
- Wolf P. The nature and significance of platelet products in human plasma. Br J Haematol. 1967;13(3):269–288. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

