Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity
- PMID: 31983316
- PMCID: PMC7524152
- DOI: 10.1080/19490976.2020.1714401
Dietary restriction increases protective gut bacteria to rescue lethal methotrexate-induced intestinal toxicity
Abstract
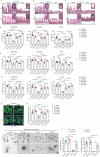
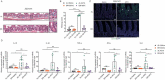
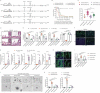
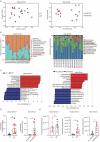
Methotrexate (MTX) is a typical chemotherapeutic drug that is widely used in the treatment of various malignant diseases as well as autoimmune diseases, with gastrointestinal toxicity being its most prominent complication which could have a significant effect on the prognosis of patients. Yet effective ways to alleviate such complications remains to be explored. Here we show that 30% dietary restriction (DR) for 2 weeks dramatically increased the survival rate of 2-month-old female mice after lethal-dose MTX exposure. DR significantly reduced intestinal inflammation, preserved the number of basal crypt PCNA-positive cells, and protected the function of intestinal stem cells (ISCs) after MTX treatment. Furthermore, ablating intestinal microbiota by broad-spectrum antibiotics completely eliminated the protective effect achieved by DR. 16S rRNA gene deep-sequencing analysis revealed that short-term DR significantly increased the Lactobacillus genus, with Lactobacillus rhamnosus GG gavage partially mimicking the rescue effect of DR on the intestines of ad libitum fed mice exposed to lethal-dose MTX. Together, the current study reveals that DR could be a highly effective way to alleviate the lethal injury in the intestine after high-dose MTX treatment, which is functionally mediated by increasing the protective intestinal microbiota taxa in mice. Keywords: Dietary restriction, Methotrexate, Gut microbiota, Intestinal stem cells, intestinal toxicity.
Keywords: Dietary restriction; gut microbiota; intestinal stem cells; intestinal toxicity; methotrexate.
Figures


References
-
- Bookbinder SA, Espinoza LR, Fenske NA, Germain BF, Vasey FB. Methotrexate: its use in the rheumatic diseases. Clin Exp Rheumatol. 1984;2:185–193. PMID:6398173. - PubMed
-
- Toffoli G, Russo A, Innocenti F, Corona G, Tumolo S, Sartor F, Mini E, Boiocchi M. Effect of methylenetetrahydrofolate reductase 677C–>T polymorphism on toxicity and homocysteine plasma level after chronic methotrexate treatment of ovarian cancer patients. Int J Cancer. 2003;103:294–299. PMID:12471611. doi: 10.1002/ijc.10847. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
