Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation
- PMID: 31983508
- PMCID: PMC7176020
- DOI: 10.1016/j.molcel.2020.01.003
Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation
Abstract
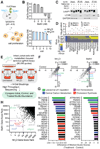
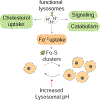
The lysosome is an acidic multi-functional organelle with roles in macromolecular digestion, nutrient sensing, and signaling. However, why cells require acidic lysosomes to proliferate and which nutrients become limiting under lysosomal dysfunction are unclear. To address this, we performed CRISPR-Cas9-based genetic screens and identified cholesterol biosynthesis and iron uptake as essential metabolic pathways when lysosomal pH is altered. While cholesterol synthesis is only necessary, iron is both necessary and sufficient for cell proliferation under lysosomal dysfunction. Remarkably, iron supplementation restores cell proliferation under both pharmacologic and genetic-mediated lysosomal dysfunction. The rescue was independent of metabolic or signaling changes classically associated with increased lysosomal pH, uncoupling lysosomal function from cell proliferation. Finally, our experiments revealed that lysosomal dysfunction dramatically alters mitochondrial metabolism and hypoxia inducible factor (HIF) signaling due to iron depletion. Altogether, these findings identify iron homeostasis as the key function of lysosomal acidity for cell proliferation.
Keywords: CRISPR; Chelation; Genetic Screens; Iron Depletion; Iron Homeostasis; Iron Sulfur Clusters; Lysosomal Acidity; Lysosomal Dysfunction; Organelle Metabolism; v-ATPase.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




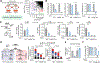
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

