Thermal Stability and Explosive Hazard Assessment of Diazo Compounds and Diazo Transfer Reagents
- PMID: 31983869
- PMCID: PMC6972035
- DOI: 10.1021/acs.oprd.9b00422
Thermal Stability and Explosive Hazard Assessment of Diazo Compounds and Diazo Transfer Reagents
Abstract
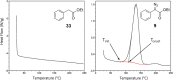
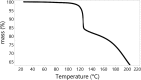
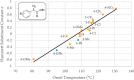
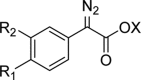
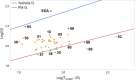
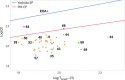
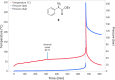
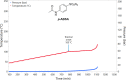
Despite their wide use in academia as metal-carbene precursors, diazo compounds are often avoided in industry owing to concerns over their instability, exothermic decomposition, and potential explosive behavior. The stability of sulfonyl azides and other diazo transfer reagents is relatively well understood, but there is little reliable data available for diazo compounds. This work first collates available sensitivity and thermal analysis data for diazo transfer reagents and diazo compounds to act as an accessible reference resource. Thermogravimetric analysis (TGA), differential scanning calorimetry (DSC), and accelerating rate calorimetry (ARC) data for the model donor/acceptor diazo compound ethyl (phenyl)diazoacetate are presented. We also present a rigorous DSC dataset with 43 other diazo compounds, enabling direct comparison to other energetic materials to provide a clear reference work to the academic and industrial chemistry communities. Interestingly, there is a wide range of onset temperatures (T onset) for this series of compounds, which varied between 75 and 160 °C. The thermal stability variation depends on the electronic effect of substituents and the amount of charge delocalization. A statistical model is demonstrated to predict the thermal stability of differently substituted phenyl diazoacetates. A maximum recommended process temperature (T D24) to avoid decomposition is estimated for selected diazo compounds. The average enthalpy of decomposition (ΔH D) for diazo compounds without other energetic functional groups is -102 kJ mol-1. Several diazo transfer reagents are analyzed using the same DSC protocol and found to have higher thermal stability, which is in general agreement with the reported values. For sulfonyl azide reagents, an average ΔH D of -201 kJ mol-1 is observed. High-quality thermal data from ARC experiments shows the initiation of decomposition for ethyl (phenyl)diazoacetate to be 60 °C, compared to that of 100 °C for the common diazo transfer reagent p-acetamidobenzenesulfonyl azide (p-ABSA). The Yoshida correlation is applied to DSC data for each diazo compound to provide an indication of both their impact sensitivity (IS) and explosivity. As a neat substance, none of the diazo compounds tested are predicted to be explosive, but many (particularly donor/acceptor diazo compounds) are predicted to be impact-sensitive. It is therefore recommended that manipulation, agitation, and other processing of neat diazo compounds are conducted with due care to avoid impacts, particularly in large quantities. The full dataset is presented to inform chemists of the nature and magnitude of hazards when using diazo compounds and diazo transfer reagents. Given the demonstrated potential for rapid heat generation and gas evolution, adequate temperature control and cautious addition of reagents that begin a reaction are strongly recommended when conducting reactions with diazo compounds.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Zhang T. Y. Process Chemistry: The Science, Business, Logic, and Logistics. Chem. Rev. 2006, 106, 2583–2595. 10.1021/cr040677v. - DOI - PubMed
- Butters M.; Catterick D.; Craig A.; Curzons A.; Dale D.; Gillmore A.; Green S. P.; Marziano I.; Sherlock J.; White W. Critical Assessment of the Pharmaceutical Processes – A Rationale for Changing the Synthetic Route. Chem. Rev. 2006, 106, 3002–3027. 10.1021/cr050982w. - DOI - PubMed
-
- Stoessel F.Thermal Safety of Chemical Processes; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2008.
-
-
For recent examples of studying hazardous properties of reagents or reactions, see:
- Sperry J. B.; Minteer C. J.; Tao J.; Johnson R.; Duzguner R.; Hawksworth M.; Oke S.; Richardson P. F.; Barnhart R. W.; Bill D. R.; Giusto R. A.; Weaver J. D. Thermal Stability Assessment of Peptide Coupling Reagents Commonly Used In Pharmaceutical Manufacturing. Org. Process Res. Dev. 2018, 22, 1262–1275. 10.1021/acs.oprd.8b00193. - DOI
- Richardson M. B.; Brown D. B.; Vasquez C. A.; Ziller J. W.; Johnston K. M.; Weiss G. A. Synthesis and Explosion Hazards of 4-Azido-l-phenylalanine. J. Org. Chem. 2018, 83, 4525–4536. 10.1021/acs.joc.8b00270. - DOI - PMC - PubMed
- Alazet S.; Preindl J.; Simonet-Davin R.; Nicolai S.; Nanchen A.; Meyer T.; Waser J. Cyclic Hypervalent Iodine Reagents for Azidation: Safer Reagents and Photoredox-Catalyzed Ring Expansion. J. Org. Chem. 2018, 83, 12334–12356. 10.1021/acs.joc.8b02068. - DOI - PubMed
- Dallaston M. A.; Bettencourt C. J.; Chow S.; Gebhardt J.; Spangler J.; Johnston M. R.; Wall C.; Brusnahan J. S.; Williams C. M. Ranking Oxidant Sensitiveness: A Guide for Synthetic Utility. Chem. – Eur. J. 2019, 25, 9614–9618. 10.1002/chem.201902036. - DOI - PubMed
-
-
-
For comprehensive works on energetic materials, terminology and their characterization see
- Klapötke T. M.Chemistry of High-Energy Materials, 3rd ed.; Walter de Gruyter GmbH: Berlin, 2015.
- Matyáš R.; Pachman J.. Primary Explosives, 1st ed.; Springer-Verlag: Berlin, Heidelberg, Germany, 2012.
-
-
- Nefati H.; Diawara B.; Legendre J. J. Predicting the Impact Sensitivity of Explosive Molecules Using Neuromimetic Networks. SAR QSAR Environ. Res. 1993, 1, 131–136. 10.1080/10629369308028824. - DOI - PubMed
- Tian B.; Xiong Y.; Chen L.; Zhang C. Relationship between the Crystal Packing and Impact Sensitivity of Energetic Materials. CrystEngComm 2018, 20, 837–848. 10.1039/C7CE01914A. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
